The chemical compound XeF4 (Xenon Tetrafluoride) comprises Xenon and Fluoride atoms. It is the first binary chemical found in the world. It is a noble gas with the chemical formula
Xe + 2F2 -> XeF4.
In solid form, the XeF4 has a density of 4.040 g cm3 and has a solid white look. It is 115.7 degrees Celsius (240.26 degrees Fahrenheit) in temperature. The Xenon Tetrafluoride, like the other Xenon Fluorides, exhibits an exergonic formation. At 115.7 degrees Celsius, the chemical sublimates. When exposed to normal pressure and temperature, XeF4 remains stable.
It maintains its stability at typical temperatures and pressures. It produces molecular oxygen, hydrogen fluoride, and pure xenon gas after coming in contact with water.
Lewis Structure of XeF4
Now that we know the valence electrons of Xenon Tetrafluoride, sketching its Lewis structure will be much easier. Lewis dot structure shows the relationship between valence electrons surrounding specific atoms in a molecule. Lines denote the bonds in the structure, whereas dots denote the electrons not engaged in bond formation. Nonbonding electrons, also known as lone pairs of electrons, are electrons that do not form any bonds with other electrons.
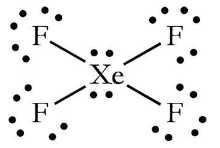
We will put Xenon in the middle, with all the other fluorine atoms around it, since it is the least electronegative atom. Each molecular link absorbs two electrons, and since this molecule has four single bonds, eight of the 36 electrons are consumed. Begin wrapping the valence electrons around the atoms with the remaining valence electrons. One electron from the outer valence electron connected the fluorine atom to the outer valence electron.
Observe that 24 electrons have already been attached to the fluorine atoms. Due to its exception from the octet rule, Xenon will become the recipient of the lone electron pairs of nonbonding electrons when atoms are atomised. Xenon combines these nonbonding electron pairs with these two nonbonding electron pairs to form a Lewis structure with two pairs of electrons lone on Xe and six nonbonding electrons on each Fluorine atom.
XeF4 Molecular Geometry
The geometry of molecules, also known as molecular structure, is a three-dimensional representation of the complete molecule. It is a helpful idea for comprehending and analysing reactivity, polarity, colour, phase of matter, magnetism, and other phenomena. However, the Lewis structure hypothesis does not account for a molecule’s form. As a result, molecular geometry is used to establish the fundamental form of the molecules.
In the end, the Lewis dot structure reveals the unpaired electrons or lone pairs. There may be electrical repulsion between these two pairs of nonbonding electrons since they are present in such a manner. As a result, the VSEPR hypothesis implies that there must be a minimum of electron repulsion. This will aid in the formation of a stable structure for the chemical. It focuses on the locations achieved by groups of electrons on a molecule’s core atom. The locations may be accurately predicted by seeing all of the groupings of electrons, whether they are bonding or nonbonding pairs of electrons.
A correct Lewis structure must be created to get at a molecule’s geometry. It aids in determining the number of bonding and nonbonding electron groups. Nonbonding electrons are arranged on a perpendicular plane inside an octahedral arrangement to achieve a stable structure. They are 180 degrees apart and face the other direction. It is the only cause behind xenon tetrafluoride’s square planar form.
XeF4 Hybridisation
It is how an atom’s orbitals merge to generate a new hybridised orbital, which gives molecules their shape and unique bonding characteristics. It also symbolises the valence bond hypothesis. Quantum mechanics explains the concept of hybridisation. Atomic orbitals on the same level are permitted to participate in the process. The energy is reallocated to the other orbitals to provide comparable energy. Hybrid orbitals are orbitals that have been created recently. It only happens when atoms are in the process of forming bonds, not when they are in their gaseous condition.
An atom’s two orbitals of the same energy level fuse together to generate a new kind of orbital. The hybridisation of the molecule XeF4 will show here. Xenon Tetrafluoride comprises the core atom xenon, which serves as the epicentre for hybridisation. The atom’s valence shell has 6 electrons in the 5p orbital and 2 electrons in the 5s orbital. In the 5th shell, there are no electrons in the f and d orbitals. To fill the empty spaces in the 5d orbitals, two electrons from the 5p orbital jump up to the 5d orbitals. They are excited. The sp3d2 hybridisation, which has two unpaired electrons in the 5p orbital and two others in the 5d orbital, is formed by the remaining four unpaired electrons. During the process, a sigma bond is formed.
XeF4 Bond Angles
F-Xe-F bonds have 90-degree bond angles, whereas lone pairings have 180-degree angles. Because the fluorine atoms are at 90 degrees to one another, the electrons in the molecule’s plane are distributed symmetrically. These bond angles aid the creation of square planar molecular geometry.
In XeF4 Polarity
XeF4 is a non-polar chemical. Because XeF4 has a symmetrical geometrical structure, it is square planar. The dipoles across the Xe-F bond cancel each other out, resulting in a zero net dipole. As a result, the XeF4 molecule has a homogeneous charge distribution with no polarisation.
Conclusion
Because it has a simple structure, Xenon Tetrafluoride is a basic molecule. The dipole moment inside the bonds is zero, making it a non-polar molecule. The nonbonding electrons produce an octahedral shape in the structure, which is square planar. The lone pairs occupy the axial locations of the geometry to reduce repulsion in an octahedral form.
Related Links:
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





