Swedish mineralogist, Axel Fredrik Cronstedt coined the term “zeolite” in the year 1756. Large amounts of steam were produced during the rapid heating of a material that was found to be stilbite. This was due to the water that had been absorbed by the material previously. He coined the term zeolite after witnessing such events. It is derived from the Greek words (zé), which means “to boil,” and (lthos), which means “stone.”
Compounds of silicone
The most abundant compounds in the earth’s crust are silica and silicates (around 95 percent ). Silicon dioxide (SiO2), also known as silica, exists in a variety of crystallographic forms. Quartz, cristobalite, and tridymite are crystalline forms of silica that are interconvertible when heated to the right temperature. Silicon dioxide is a covalent compound that is tetrahedrally attached to four oxygen atoms.
SiO2 has a massive structure, with alternate oxygen and silicon atoms forming an eight-membered ring. The Si-O bond is unreactive to many acids, halogens, and alkalies due to its high bond enthalpy, but it dissolves in HF and NaOH.
Silica gel, a drying agent, also aids in the support of chromatographic materials and catalysts.
Silicones
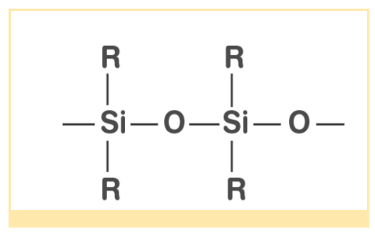
It refers to a family of organosilicon polymers with repeating units. Silicones are made from alkyl or aryl substituted silicones as the starting material. Silicones, as we all know, are surrounded by a non-polar alkyl group that repels water in nature. They have a high thermal stability and are oxidation and chemical resistant. As an electrical insulator and in surgeries, this compound has a wide range of applications.
Silicates
Silicates, such as zeolites, come in a variety of forms. Silicate has the structure SiO44-, with four oxygen atoms attached to one silicon atom. When silicate units are joined together, they form a ring, a chain, and a three-dimensional structure. Glass and cement are two important man-made silicates.
Zeolites
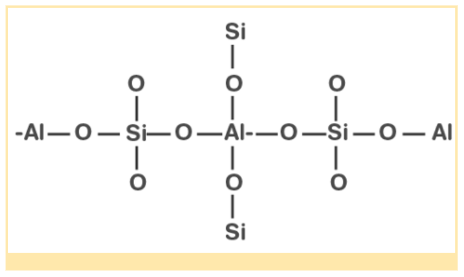
When some silicon atoms are displaced by aluminium atoms in the three-dimensional structure of silicon dioxide, aluminosilicate is formed. In the petrochemical industry, zeolites are primarily used as catalysts. Using a zeolite called ZSM-5, alcohols can be converted directly to gasoline. These zeolites can also be used to soften hard water.
Types of Zeolites
Zeolites come in a variety of shapes and sizes. Zeolites can be synthesised or manufactured industrially, or they can be formed naturally. There are currently 50 different types of zeolites available.
Zeolites are found in a variety of forms, including:
- Na2K2CaMg(AlO2)2(SiO2)2.6H2O (Erionite)
- Na2Ca(AlO2)2(SiO2)4.6H2O (Gemelinite)
- Hx[(AlO2)x(SiO2)96−x].16H2O (ZSM-5)
Properties of zeolites
- Under a variety of environmental conditions, zeolites are extremely stable solids. Zeolite has a very high melting point of 1000 0 C. They are not soluble in water or other inorganic solvents.
- Zeolite is known for its open cage-like framework structure, which aids in the trapping of water and potassium and calcium ions.
- Natural zeolites are found in a variety of shapes and sizes, with pore sizes that are not uniform, whereas synthetic zeolites are made with precision and pore sizes that are uniform.
- Alumina-rich zeolites are attracted to polar molecules like water, whereas silica-rich zeolites are attracted to nonpolar molecules.
Occurrence and production
As previously stated, zeolites occur naturally in areas where alkaline groundwater reacts with volcanic rocks and ash layers. According to the reports, approximately 245 unique zeolite frameworks have been discovered, with approximately 40 naturally occurring zeolite frameworks known. The International Zeolite Association Structure Commission carefully examines any new zeolite structure that is discovered. The material is assigned a three-letter designation after it has been identified.
Meanwhile, industrially important zeolites are manufactured synthetically. Heating aqueous solutions of alumina and silica with sodium hydroxide is one of the most common procedures used. The reagents sodium aluminate and sodium silicate are also interchangeable. Changes in the cations, such as adding quaternary ammonium cations, are another variation. Over 200 synthetic zeolites have been created to date. This was accomplished through a slow crystallisation process involving silica-alumina gel, alkalis, and organic templates.
Synthetic zeolites, on the other hand, have few advantages over natural zeolites. Synthetic zeolites are manufactured in a phase-pure and uniform state. In addition, industrially produced unique zeolite structures are possible. Zeolite A, for example. Furthermore, because silica and alumina are the most abundant mineral components on the planet, zeolites can be manufactured and distributed indefinitely.
Application of zeolites
The following are some of the most common applications for zeolites.
Ion exchange
The cage-like structure of zeolites makes them ideal for ion exchange. Hard water, for example, is filtered through a column of sodium-containing zeolites. Calcium and magnesium ions are trapped by zeolites, while sodium ions are released, resulting in water softening and sodium enrichment. Nowadays, zeolites are used in detergent to remove magnesium and calcium from the water, making it softer and increasing the detergent’s effectiveness.
As Catalyst
Cracking, isomerization, and hydrocarbon synthesis are all reactions in which zeolites are used as a catalyst. Zeolite is a highly effective catalyst due to its porous structure. Furthermore, because the pores in a particular zeolite are of a fixed shape and size, zeolite is sometimes referred to as shape-selective catalysis because it is selective on specific molecules.
Adsorbent
Zeolites are used to adsorb a wide range of materials due to their high adsorption capacity. In the fields of purification, drying, and separation, they have a wide range of applications.
Getting Rid of Harmful Substances
Radioactive particles can be effectively removed from nuclear waste using zeolites. It can also be used to clean heavy toxic metal-contaminated water or soil.
Commercial and Residential
- Cryosorption vacuum pumps frequently use zeolites as a molecular sieve.
- Synthetic zeolites are used as an additive in the warm mix asphalt concrete manufacturing process.
Gemstones
Thomsonites, one of the rarer zeolite minerals, are prized as gemstones.
Biological
- Medical-grade oxygen is commonly produced using zeolite-based oxygen concentrator systems.
- In agriculture, clinoptilolite (a naturally occurring zeolite) is used as a soil treatment.
Solar Energy Storage and Application
Thermochemically storing solar heat captured from solar thermal collectors has been done with zeolites. It can also be used for adsorption cooling. The high heat of adsorption and ability to hydrate and dehydrate while maintaining structural stability of zeolites are used extensively in such applications. Natural zeolites are very useful in harvesting solar and waste heat energy because of their hygroscopic property, which is combined with an inherent exothermic reaction during the transition from a dehydrated to a hydrated state.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





