Beryllium is a grey metal used as a hardening agent in metallurgy. Beryllium chloride is made of beryllium and chloride. It is carcinogenic and used as genotoxin. Hybridization is the process of creating a new hybrid structure combining an element’s atomic orbital; the formed hybrid will have a different shape and energy. Beryllium chloride and tin chloride have a similar molecular formula. Beryllium dichloride is sp hybridised and it has a linear structure, whereas tin chloride has a bent structure. Let’s study the hybridization and its characteristics along with beryllium dichloride hybridization.
Hybridization
Hybridization is the process of creating a new hybrid structure combining an element’s atomic orbital; the formed hybrid will have a different shape and energy. Compounds with the same molecular formula might have different structures due to the difference in atomic orbital they filled. It is established that the lowest energy configuration for an atom is the one having the maximum number of unpaired electrons allowed by the Pauli principle in a set of orbits with the same energy. Hybridization can explain a molecular shape. Based on the types of orbital, the hybridization can be sp3, sp2, sp, sp3d, sp3d2, sp3d3.
Sp hybridization
It is a hybridization in which 50 % of ’s’ and 50% of ‘p’ characters are shown. It is the mixing of one s and one p orbital of equal energy. It is also called diagonal hybridization. Examples include BeCl₂, BeF₂, BeH₂ and all carbon compounds having a triple bond.
Beryllium dichloride
Beryllium dichloride is a colourless inorganic compound. It is used as a raw material in beryllium electrolysis and catalyst in the Friedel-Crafts reaction. Beryllium oxide is carbon thermally reduced to form beryllium chloride.
Beryllium chloride hybridization – notes
Beryllium has two valence electrons and its electron configuration is (1s2 2s2). One of its 2s electrons was promoted to empty 2p orbital. Thus, in the excited state, the electronic configuration of ‘Be’ is 1s2 2s1 2p1. Beryllium must be angular if it is formed with its pure orbital. But in the excited state, the beryllium atom undergoes ‘sp’ hybridization by mixing 2s and one 2p orbital. These two half-filled orbitals form two σ bonds with chlorine. Therefore BeCl2 is necessarily linear, since two sp hybridised orbital forms have a bond angle of 180°.
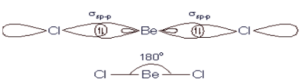
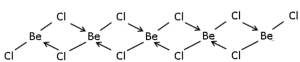
But in the solid-state, beryllium dichloride exists as a polymer. Therefore one beryllium forms a covalent bond with two chlorine and coordinates bonds with two chlorine. It exhibits sp3 hybridization and it is a polymeric chain in structure.
Beryllium dichloride hybridization – questions
What is the hybridization of beryllium dichloride in a gaseous state?
Beryllium has two valence electrons and its electron configuration is (1s2 2s2). One of its 2s electrons was promoted to empty 2p orbital. Thus in the excited state, the electronic configuration of ‘Be’ is 1s2 2s1 2p1. Beryllium must be angular if it is formed with its pure orbital. But In the excited state, the beryllium atom undergoes ‘sp’ hybridization by mixing 2s and one 2p orbital. These two half-filled orbitals form two σ bonds with chlorine. Therefore BeCl2 is necessarily linear since two sp hybridised orbital forms have a bond angle of 180°.
What is the hybridization of beryllium dichloride in solid state?
But in the solid-state, beryllium dichloride exists as a polymer. Therefore one beryllium forms a covalent bond with two chlorine and coordinate bonds with two chlorine. It exhibits sp3 hybridization and it is a polymeric chain in structure.
Draw structure of beryllium dichloride in the gaseous state.

Draw structure of beryllium dichloride in the solid-state.

Conclusion
Beryllium dichloride is a colourless inorganic compound. It is used as a raw material in beryllium electrolysis and catalyst in the Friedel-Crafts reaction. Beryllium oxide is carbon thermally reduced to form beryllium chloride. Beryllium must be angular if it is formed with its pure orbital. But in the excited state, the beryllium atom undergoes ‘sp’ hybridization by mixing 2s and one 2p orbital. These two half-filled orbitals form two σ bonds with chlorine. Therefore BeCl2 is necessarily linear since two sp hybridised orbital forms have a bond angle of 180°. But in a solid-state, beryllium dichloride exists as a polymer. Therefore one beryllium forms a covalent bond with two chlorine and coordinate bonds with two chlorine. It exhibits a sp3 hybridization and it is a polymeric chain in structure.
Related Links:
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




