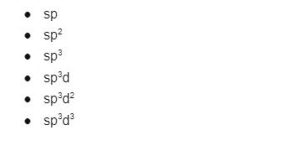Introduction
In chemistry, hybridisation is explained as combining two atomic orbitals to create a new type of hybridised orbitals. The development of a hybrid orbital with completely different shapes, energies and so on is frequently the outcome of this intermixing. Atomic orbitals mostly carry out hybridisation with the same energy level. However, filled & partially filled orbitals can both participate in the mentioned process if their energies are equivalent.
Alternatively, the hybridisation theory can be viewed as a valence bond theory’s extension (VSEPR), as it aids in understanding the bond formation, bond lengths, and bond energy.
What are the different types of Hybridisation?
In 1931, scientist Pauling discovered the remarkable theory of hybridisation in the year 1931. He explained it as the shifting of energy of the orbital of particular atoms to give the orbitals of the equivalent energy and called the process hybridisation. New orbitals, called hybrid orbitals, emerge due to this process.
Criteria for determining the type of Hybridisation
To determine the type of hybridisation, you must have to follow the given rules:
- Figure out the total no. of the valence electron.
- Calculate the total number of the lone pairs of electrons or calculate the total octet or duplex.
- Determine used orbital = no. of octet or duplex + Number of single-electron pairs.

sp or Diagonal Hybridisation
sp hybridisation, usually known as diagonal hybridisation, occurs when two s & one p orbitals belonging to the same primary shell of an atom combine to generate two new identical hybrid orbitals. The molecule generated due to this hybridisation has a linear shape with an angle of 180 degrees. The resulting hybrid orbitals contain 50% s and 50%p characters.
In the bond formation of compound BeH2:
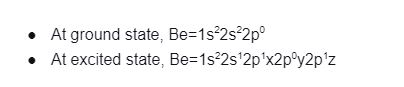
sp2 or Trigonal Hybridisation
This sort of hybridisation is known as sp2 and occurs when one s and two p orbitals from the same main shell of an atom combine to generate three new equivalent hybrid orbitals. It is also known as the Hybridisation of Trigonal Hybridization. After its hybridisation, the molecule takes on the shape of a triangle planner with a 120-degree angle. The hybrid orbitals make approx. 33.33% s character & 66.66% p character.
In the bond formation of compound BH3:

sp3 or Tetrahedral Hybridisation
When a single p orbital goes into the energy mixing process to make a new orbital, such a kind of hybridisation is called sp hybridisation. The molecules possessing sp hybridisation used to have a linear shape with an angle of 180°. The molecule formed due to this hybridisation is tetrahedral, with an angle of 109o28′. About 25% of the hybrid orbitals generated have s character, and 75% have p character.
Sp3d or trigonal bipyramidal
sp3d hybridisation contains 1 ‘s’, 3 ‘p’ and 1 ‘d’ orbitals. All of these have the same energy level, giving five degenerate and identical hybrid orbitals.
sp3d2 or octahedron
sp3d2 hybridisation contains 1′ s’, 3′ p’ and 2 ‘d’ orbitals which undergo intermixing to make six similar sp3d2 hybrid orbitals.
Conclusion
In the above chapter, we have understood the different concepts related to hybrid orbital and various configurations such as sp, sp2 sp3, sp3d, sp3d2 Hybridised Orbitals. Hybridisation is a crucial topic as all of the elements in our environment behave in unusual and unexpected ways.
Related Links:
The electrical components of these elements and their properties are interesting topics to study. We can draw various practical uses of such elements because of the uniqueness of their features and usage. When it comes to the elements in our environment, we can see a wide range of physical qualities. The subject of hybridisation, or how it enables the unique combination of distinct molecules, is crucial in science.
Different elements make different shapes, such as Linear, trigonal planar, tetrahedral, trigonal bipyramidal, and octahedral.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out