Several chemical reactions do not complete their reaction chain. Instead, they attain a state of chemical equilibrium. This is a state in which the rates of the forward and reverse reactions are equal. Plus, all the reactants and products in a concentration remain constant (the values don’t change).
Equilibrium is a dynamic process where there is a constant conversion of reactants to products or vice versa. In this process, the exchanges occur without any net change in the product molecules or the number of reactants. In this article, we will talk about applications of equilibrium constant.
Key Differences between Kc and Kp
Kc and Kp are known as equilibrium constants. These constants in a reaction mixture represent the ratio between the pressure of products and concentration in a chemical reaction. The main difference is that Kc is expressed in terms of concentration, whereas Kp is expressed as pressure. These equilibrium constants are used for reversible reactions and in all applications of equilibrium constants.
Kc can also be defined as the relationship between the concentration of products and reactants.
Kp is the ratio between the pressure of reactants and products.
What is Kc?
Kc is the ratio of the concentration of products and reactants in chemical equilibrium. The components used to express the molar concentrations are known as Kc.
aA + bB ↔ cC + dD
This reaction can be written as:
Kc = [C]c [D]d[A]a [B]b
Here, the constants a and b refer to the concentration of reactants, whereas c and d are its products. These constants are called stoichiometric coefficients of products and reactants in chemical equilibrium. In terms of Kc, the stoichiometric coefficients are raised to their powers to equal concentrations of reactants and products.
The equilibrium constants may vary in terms of reactants and products, but their Kc is always the same. In applications of the equilibrium constant, the Kc is also the same.
Other features of Kc
Equilibrium can be obtained from different directions
The value of Kc does not depend on the initial concentration of products and reactants, but it depends on temperature
The magnitude of Kc
If the value of Kc is big, then Kc >> 1. Then, the equilibrium is present in the right, and the reaction mixture is primarily made of products.
But if the value of Kc is small, Kc << 1. Then, the equilibrium is present on the left side, and the mixture comprises reactants.
Now in the third condition, if the value is close to 1, then it is (0.10 < Kc < 10). The mixture has an equal amount of reactants and products.
What is Kp?
Kp can be defined as the ratio between the presence of products and reactants. This constant is used in gaseous mixtures. The value of Kp depends on the partial pressure of the gaseous state in the reaction.
P + qQ ↔ rR + sS
In this equation, the equilibrium constant can be written as:
Kp = Rr . SsPp . Qq
Where is partial pressure. Therefore, P, Q, R, and S represent partial pressures of P, Q, R, and S gas components in the above equation. Exponents p, q, r, and s are stoichiometric coefficients of products and reactants in a gaseous mixture.
Important points to remember while writing a Kp equation
Even though both-sided arrows (⇌) are included in equilibrium equations, we treat left side elements as reactants and right side as products.
All the products are on the top of the numerator in the equilibrium, whereas the reactants sink to the bottom of the container and become the denominator.
The partial pressure is raised to its power and is equal to the stoichiometric coefficients of the substance in the balanced equation.
In the heterogeneous mixture, the partial pressure of pure solids and liquids are not included.
Relationship between these two equilibrium constants
Here are more notes on the relationship between Kc and Kp.
This relation between Kc and Kp can be defined using the equation:
Kp = Kc(RT)Δn
Kp denotes the pressure equilibrium constant, Kc denotes the concentration equilibrium constant, R is the universal gas constant (8.314 Jmol-1K-1), T is the temperature, and n is the difference between total moles of gas products and total moles of gas reactants.
Example: Find the equilibrium constant between silver and copper ions.
Cu(s) + 2Ag+ ⇆ Cu2+(aq) + 2Ag(s)
This equation can be written as:
Kc = [Cu2+] / [Ag+]2
In this equation, the solid silver and copper are eliminated. Also, the coefficient for the copper ions is converted into an exponent after the equilibrium calculation is done.
Relationship between the two constants in gaseous mixtures
We can assume that all the gases present in the equilibrium obey the ideal gas law. Then the partial pressure (p) present in the mixture of gas would be
PV = nRT
Dividing both the sides by V
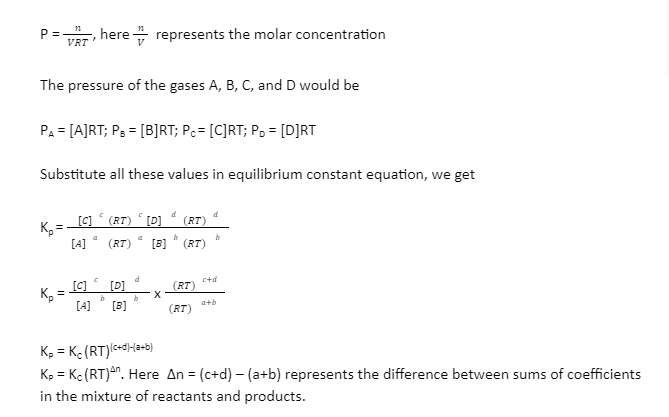
You can equate that ∆n = 0, Kp = Kc from the above equation.
Kc Vs. Kp
Difference between Kc and Kp | |
Kc | Kp |
It can be defined as a ratio between the concentration of products and reactants | This equilibrium constant is for the ratio between the pressure of products and reactants |
This constant can be used for both gaseous reactions and liquids | This constant can only be used for gaseous mixtures |
It is defined by units of concentration | It is defined by units of pressure |
Conclusion
An equilibrium constant is a mathematical expression that specifies the relationship between reactant and product concentrations in a reversible chemical reaction. The equilibrium constant is always a ratio of equilibrium concentrations, and it doesn’t depend on the absolute concentrations of anything. It does, however, depend on temperature Kp and Kc are the gaseous mixture equilibrium constants in a reversible reaction, and they are directly proportional to each other as shown by the equation Kp = KC(RT)Δng.
Related Links:
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





