D-block elements are those that have an incompletely filled d-subshell in their lowest energy state or most stable oxidation state.
They are also known as transition elements.
The (n-1) d subshell is included in the partially filled subshells.
In the farthest shell, all d-block elements have about the same number of electrons.
As a result, they have similar chemical properties.
Alloy formation, high melting point, density, atomic and ionic radii, and typical metallic properties are among the physical properties of d block elements. (n-1) (d0-10) n(s1-2) represents the electronic configuration of d-block elements. d- block elements can be stable in either a half-filled orbital or a fully filled orbital.
D-block/transition elements
The d block, also referred to as transition metals, is found between the s and p blocks. These elements have the name d block elements because the last electron of these elements enters the very last d subshell. The d-block elements are located in the periodic table between the s-block and p-block elements. Since they display transitional behaviour between s-block and p-block elements, these d-block elements are known as transition elements.
Their characteristics are intermediate between highly reactive metallic elements of the s-block, which are typically ionic compounds, and elements of the p-block, which are mostly covalent.
Some Properties of d-block/transition elements.
- Reactivity: As we move from the top left to the bottom right corner of the d block, electronegativities increase overall, densities and electrical and thermal conductivities increase, and metal cation enthalpies of hydration decrease in magnitude.
- The reaction rate increases as the energy of reactant activation decreases. This decline is caused by the catalyst, which most likely alters the reaction path.
- Except for the first and last members in the series, all of the transition elements exhibit various oxidation states.
- From the first transition series elements in the groups of transition elements, the size of atoms and ions increases. Every time a new shell is added, the atomic and ionic size increases from top to bottom.
- In their solid or liquid states, the majority of d-block metal compounds are coloured.
What is a Transition Element?
Transition elements are chemical elements that have at least one stable cation with partially filled d orbitals. Many transition elements have atoms with incomplete d orbitals, and many of them form cations with unpaired electrons in d orbitals.
Examples :
Titanium (Ti) = [Ar]3d24s2 = Ti+2 = [Ar]3d24s0
Vanadium (V) = [Ar]3d34s2 = V+3 = [Ar]3d24s0
Some d block elements are not considered to be transition elements. This is due to the fact that they do not form cations with incomplete d orbitals. The normal atom could have unpaired d electrons at times, however the only stable cation it produces may not have incomplete d orbital filling.
All transition elements are found in the periodic table’s d block. At room temperature, transition elements are metals that are solids. The majority of them form cations with varying oxidation states. The compounds formed by incorporating transition metals are extremely colourful.
These transition metals are catalytic in nature. As a result, they serve as catalysts in chemical reactions. Because of the large number of unpaired electrons, mostly all transition elements can be either paramagnetic or ferromagnetic.
Relationships and Differences between d-block elements and transition elements
Given below are some differences between d-block elements and transition elements :
- D-block elements are chemical elements that have electrons in their d orbitals. Whereas, transition elements are chemical elements that have at least one stable cation that has partially filled d orbitals.
- Colourful complexes can be formed by D block elements or not. Colourful complexes are formed by transition elements at all times.
- Many d block elements are diamagnetic, while some are paramagnetic or ferromagnetic. All transition elements are paramagnetic or ferromagnetic.
- Many d-block elements are not solids at room temperature (mercury is a liquid), but others are, whereas all transition metals are solids at room temperature.
- Several D-block elements exhibit multiple oxidation states, while others exhibit a single oxidation state, and Transition elements exhibit multiple oxidation states.
Relationships between D-block elements and transition elements :
- D block elements are all transition elements, but not all transition elements are d block elements.
- The periodic table’s d block contains almost all of the transition elements.
- Both have extremely high melting and boiling temperatures.
- At room temperature, the majority of D block elements as well as all transition elements are solids.
Conclusion
The d block, also referred to as transition metals, is found between the s and p blocks. The d-block elements are located in the periodic table between the s-block and p-block elements. Some d block elements are not considered to be transition elements due to the fact that they do not form cations with incomplete d orbitals. Despite the fact that d block elements and transition elements are frequently confused, there is a distinction between the two. D block elements are used for all transition elements. However, not all d block elements are transitional. This is due to the fact that, in order to become a transition metal, all d block elements must form at least one stable cation with incomplete d orbital filling.
Related Links:
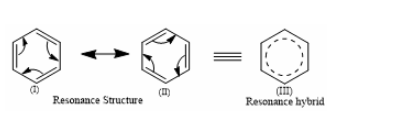
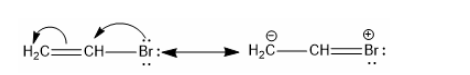
Three C-C single bonds with a bond length of 1.54 A and three C=C double bonds with a bond length of 1.34A are found in the aforementioned structures (I) and (II). However, it was discovered that all six carbon and carbon bonds are identical, and a 1.39 A intermediate C-C and C+C bond was discovered. The poor reactivity of halogen in vinyl bromide can be explained further by the phenomena of resonance.
Resonance energy is the difference between the real molecule and the more stable canonical form.
Application of resonance effect
The high utility of resonance theory and its worth comes from the fact that it maintains the simple and unsophisticated form of structural representation.
Stability of carbocation
The carbocation that conjugates a positive charge with a double bond tends to be more stable. The allylic carbocation is more stable than the comparable alkyl cation because of the resonance structure. The resonance structures are formed when the negative electrons of the conjugated double bonds are delocalised, which increases their stability. The stability will be great if the resonating structure is great.
Carbanion of stability
The availability of double bonds or an aromatic ring will enhance the anion’s stability around the negatively charged atom because of resonance.
A point to be noted: the bigger the resonance structure, the more stable it will be.
Due to resonance, the negative charge on benzyl carbanion disperses over additional carbon atoms, making it more stable than ethyl carbanion.
Stability of free radicals
Due to depolarisation of the unpaired electrons across the system, simple alkyl radicals are less stable allylic and benzylic forms of free radicals.
Mesomeric effect vs resonance effect
- Resonance effect can be defined as the process in which two or more structures can be written for the real structure of a molecule, but none of them fully explains all characteristics of molecules. Substituents or functional groups in a chemical molecule cause the mesomeric effect, denoted by the letter M.
- Delocalisation of electrons in a system is known as resonance whereas the mesomeric effect is known as the resonance effect. It is a long-term impact that is reliable on the substituents or functional groups in a chemical compound.
- The +R (electron releasing) group is equal to the +M effect, while the –R (electron attracting) group is equal to the –M effect.
Principle of resonance
- The most fundamental resonance is the one that is generated with the least charge.
- The resonance of a full octet is more substantial than that of a partial octet. The most essential forms are those in which positive charges operate on the least electronegative atom.
- The resonance structure with the greatest covalent bond is the most significant.
Resonance effect vs inductive effect
- An inductive effect occurs when the polarisation of one link is caused by another link. On the other hand, the resonance effect occurs when two or more structures may be described for molecules, but none can describe all the characteristics of a molecule on their own.
- The difference in electronegativity between two atoms in a bond affects the inductive effect directly, whereas the number of resonant structures affects the stability.
Occurrence of resonance
- A pi bond conjugated with the other pi bond
- A pi bond conjugated with a negative charge
- A pi bond with a positive charge conjugated to it
- A negative charge conjugated with the lone pair or a positive charge conjugated with a lone pair
- A pi bond conjugated with a lone pair or a free radical
Conclusion
In chemistry, resonance is an intramolecular electrical phenomenon in which the location of a pi bond(s) or a nonbonding electron changes (also called a sigma bond). In this procedure, however, the location of an atom is changed by modifying the pi electrons’ position or the non-bonding electrons’ position.
Resonance is a property of organic compounds. In organic chemistry, the delocalised electrons inside a specific compound when a single Lewis structure does not express the bond are referred to as resonance. To portray delocalised electrons in an ion or molecule, several structures known as resonance can be used.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





