Surface tension is the tendency of a fluid to contract to the smallest possible surface area. This property of the liquid is based on the fact that the molecules of liquid are in a different state at the surface than in the centre of the liquid. Surface tension is determined not only by the attractive forces between particles, but also determined by the attractive forces of solids, liquids, and gases that are in close proximity.
Surface Tension
The forces of attraction between the particles in the layer of surface and the particles in the bulk of the liquid, tending to minimize their surface area, cause surface tension. Due to surface tension, the surface of liquid allows it to oppose an external force due to the cohesive nature of its molecules.
Surface tension is given as
T=F/L
And also
T=m×a/L
Here,
T = surface tension
F = force =m×a
L = length
a = acceleration
m = mass
Unit of Surface Tension
Surface tension is determined in joules per square meter ( J/m²). Surface tension is frequently expressed as the amount of force applied to a surface which is normal (perpendicular) to a line of unit length. The surface tension is denoted by (gamma).
In SI Unit system, the surface tension is given in Nm-1 and the dimension of is kg/s². If the surface area of liquid is small, then it has the lowest energy state.
Dimension Formula of Surface tension
As we know, surface tension is
T=F/L
And also
T=m×a/L
Therefore,

Cause of Surface Tension
A molecule is pulled equally in all directions because of cohesive forces from neighbouring molecules of liquid, which results in a zero net force. Since molecules close to the surface are not surrounded by similar molecules on all sides, they are pushed inwards. This creates internal pressure which further results in liquid surfaces being compressed to the smallest possible area.
Also, due to the cohesive structure of water molecules, there is a tension which is parallel to the surface that will oppose an external force.
Cohesive and Adhesive Forces
Cohesive Force
The force which exists between the molecules of certain liquids is considered as a cohesive force. Raindrops are held together by the cohesive force before they fall to earth. Surface tension is a phenomenon which is known to us but not many of us know that surface tension is also due to the cohesive force. Surface tension allows bodies which are denser than liquids to float on them without any support and prevent them from sinking.
Adhesive Force
Adhesive force is the force of attraction between two different bodies like a solid container and a liquid. Adhesive force allows water to stick to glass.
If the phenomenon of adhesion is larger than that of cohesion, then the liquids will wet the surface of the solid with which it is in contact and we may also notice that the liquid curves towards the edge of the container. Liquids such as mercury have more cohesive force as compared to adhesive force and can therefore be defined as non-wetting liquids. Non – wetting liquids curve inward when they are near the rim of the container.
Viscosity
Viscosity is defined as the measure of resistance of flow of any fluid.
A fluid with large viscosity resists movement or motion because the intermolecular forces of fluids are strong which provides a lot of internal friction that resists the motion of the layers past one another.
On the other hand, fluid which has low viscosity can flow easily because its molecular composition produces very small friction when it moves. Gases also have viscosity, but it is difficult to detect under normal circumstances.
Viscosity of liquid decreases rapidly when temperature of liquid increases. Whereas, the viscosity of gases increases when temperature of the gas increases. Therefore, on heating, liquids flow more easily whereas the flow of gases becomes slow as compared to liquid. When the amount of matter changes then no changes occur in viscosity, thus viscosity is an intensive property.
Viscosity is the ratio of shear stress to the velocity gradient in fluid. When a sphere is dropped into a fluid, then the viscosity is determined by using the formula which is given below.
η=2g×a²×∆ρ/9v
Here,
= viscosity
g = gravitational acceleration
a = radius of sphere
∆ρ = density difference
v = velocity
Principle of viscosity
When a liquid layer is moved on a surface or other layer of the same liquid, the fluid particles tend to resist the movement and this force of resistance which is created by a liquid is known as viscosity.
Conclusion
The forces of attraction between the particles in the layer of surface and the particles in the bulk of the liquid, tending to minimize their surface area, cause surface tension.
Surface tension is given as
T=F/L
And also
T=m×a/L
Unit of surface tension is joules per square meter ( J/m²).
Dimensional formula of surface tension is
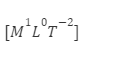
Viscosity is defined as the measure of resistance of flow of any fluid.
Viscosity is given as
η=2g×a²×∆ρ/9v
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






