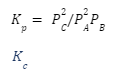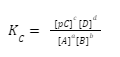The equilibrium constants of gaseous mixtures are Kc and Kp. However, Kc is determined by molar concentrations, whereas Kp is determined by the partial pressures of the gases inside a closed system. The concentrations of single components such as liquids and solids are not included in the equilibrium constants, and they may have unit factors such as the nature of the reaction (although thermodynamic equilibrium constants do not).
The value of K varies depending on whether partial pressures or concentrations are used to determine the solution. Because they are both derived from the ideal gas law, the gas equilibrium constants are related to the equilibrium (K).
Gas Equilibrium Constant
The equilibrium constants of gaseous mixtures are Kpand Kc. Kc is described in terms of molar concentrations, while Kp is expressed in terms of the partial pressure of gases present in a closed system.
Kp
Kpis the equilibrium constant determined from a reaction equation’s partial pressures. It’s a mathematical expression for the relationship between product and reactant pressures. Although it connects the pressures, it is a unitless number.
Kinetic Pulse is the definition of Kp, and more definitions may be found at the bottom of the page. Kp has three alternative definitions. When equilibrium concentrations are represented in molarity, Kpis the equilibrium constant.
Though the arrows in the equilibrium equations point both ways (⇋), we usually link the left side with the reactants and the right side with the products.
The numerator of the fraction is the numerator, and the products are the ones that are on top of it.
The reactants are the elements that make up the denominator of the percentage.
In a balanced chemical equation, the concentrations of the reactants and products are always increased to the coefficient of power.
If any of the products or reactants are solids or liquids, their concentrations are designated as one.
The equilibrium constant Kp is calculated from the partial pressure of a reaction equation. It’s a formula for calculating the relationship between product and reactant pressures. Despite the fact that it relates the pressures, it is a number without a unit.
For a given chemical reaction, aA+bB ⇌ cC+dD
Here we have mole of reactant A
b= mole of reactant B
c = mole of reactant C
d = mole of reactant D
We taking example,2Ag+Bg⇌2C(g)
We can calculate KP as:
Kc denotes the fraction of the equilibrium concentrations of products over the concentrations of reactants in a reversible reaction, where each is increased to the power of their stoichiometric coefficients.
Taking the example of a reversible reaction:cC+dD⟹aA+bB
The Kc for the above reaction are:
kp and kc
The equilibrium constants of an ideal gaseous mixture are kp and Kc. Kp is the equilibrium constant to use when representing equilibrium concentrations in atmospheric pressure, while Kc is the equilibrium constant to use when representing equilibrium concentrations in molarity. The equilibrium constant, abbreviated as k, is a quantity that describes the connection between the reactants and the number of products present at equilibrium for a reversible chemical reaction at a specific temperature.
Relation between kp and kc
Kp=Kc (RT)ng
Here n is the no of moles
And R= 0.082062 L.atomK-1 mol-1
When ng=0 means there is no change in no of gas molecules
ThenKp=Kc
Difference between kp and kc
The equilibrium constants are are Kpand Kc . The proportion between the concentrations or pressures of products and reactants in a reaction mixture is expressed by the equilibrium constant of that reaction mixture. The main distinction between are Kpand Kcis that Kcis the equilibrium constant determined by concentration terms, whereas Kp is determined by pressure terms.
For reversible reactions, the equilibrium constant is given. Kc is the equilibrium constant expressed as a ratio of product and reactant concentrations, whereas Kp is the equilibrium constant expressed as a ratio of product and reactant pressure. For gaseous or liquid reaction mixtures, Kc can be employed. Only gaseous reaction mixtures are employed with Kp. Units of concentration are used to calculate Kc. Pressure units are used to calculate Kp.
Use of kp and kc
For gaseous or liquid reaction mixtures, Kc can be employed. Only gaseous reaction mixtures are employed with Kp. The equilibrium constants of an ideal gaseous mixture are Kp and Kc. When equilibrium concentrations are represented in atmospheric pressure, Kp is the equilibrium constant to use, and when equilibrium concentrations are expressed in molarity, Kc is the equilibrium constant to use.
Conclusion
The equilibrium constants of gaseous mixtures are Kpand Kc. Kcis determined by molar concentrations, whereas Kp is determined by the partial pressures of the gases inside a closed system. Kc is described in terms of molar concentrations, while Kp is expressed in terms of the partial pressure of gases present in a closed system. The value of K varies depending on whether partial pressures or concentrations are used to determine the solution. Kpis the equilibrium constant to use when representing equilibrium concentrations in atmospheric pressure, while Kc is the equilibrium constant to use when representing equilibrium concentrations in molarity.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out