Infrared light, commonly referred as infrared radiation, is a type of electromagnetic radiation with a wavelength of up to 1050 nm. Since it is much higher than visible light, it can only be recognized by the human eye under certain circumstances.
Electromagnetic Waves
Everything in our environment, including the sun, the earth, and other bodies, emits electromagnetic energy of varied wavelengths. Electromagnetic energy travels across space in the form of sinusoidal waves at the speed of visible light.
The electromagnetic spectrum is a word that scientists use to refer to the complete range of light which exists. The wavelengths in the spectrum range from short to long. Electromagnetic (EM) waves are energy waves that are made up of oscillating electric and magnetic fields.
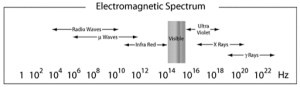
The electromagnetic spectrum’s numerous radiations are depicted in the graphic above. The wavelengths of rays on the right side of the spectrum are shorter, while those on the left have longer wavelengths. Longer wavelength radiations, like radio, microwave, and infrared, are thought to be less hazardous. Lower-wavelength radiations, like gamma, X-rays, and ultraviolet, are thought to be extremely harmful.
Infrared Radiation
One of the electromagnetic radiations in the electromagnetic spectrum is infrared radiation. It is also referred to as infrared light because of its larger wavelength, which makes it invisible to the naked eye. However, they are only detectable as a source of heat. Infrared radiation has a wavelength between 700 nm and 1 nm, that is a longer wavelength than visible light and has a frequency of 300-600 GHz.
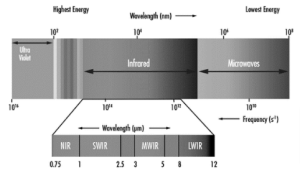
Infrared radiation emits light energy and behaves both as a wave and as a quantum particle described as a photon. Infrared radiation is classified into five types based on wavelength and frequency. Near-wavelength, short-wavelength, mid-wavelength, long-wavelength, and far-infrared are all included.
Infrared Radiation formula
The Stefan-Boltzmann law of radiation specifies the rate of heat transfer by emitted radiation.
Qt=σeAT4
Here,σ=5.67×10-8J/s.m2.k4 is the Stefan-Boltzmann constant, A is the object’s surface area and T is its absolute temperature in kelvin
Characteristics of Infrared Radiation
Infrared radiation has a variety of properties, some of which are listed below:
The wavelength of infrared light varies between 700 and 1 nm.
It operates at frequencies ranging from 430 THz to 300 GHz.
A transverse wave is infrared radiation.
They exhibit the property of refraction, which is not present in visible light.
It is caused by a change in electron movement.
It has characteristics that cause the body to heat up.
Near-infrared rays have a wavelength which is similar to visible light. They can be employed in TV remote controls, photography, and other applications.
When compared to near-infrared wavelengths, far-infrared rays are more heat.
Properties of Infrared Radiation
Infrared radiation has a number of distinct features, which are listed below:
Electronic origin
All electromagnetic radiation, including infrared light, is thought to originate at a point where the flow of electrons is changed.
Transverse waves
Transverse waves are found in other electromagnetic radiations, such as infrared. A transverse wave is defined as any displacement or waviness of the wave that is at right angles to the direction in which the wave’s energy is travelling.
Speed
According to “Serway’s College Physics,” infrared light travels at a rate of 299,792,458 m/s, much like other electromagnetic radiation.
Absorption and reflection
Infrared radiation can be absorbed or reflected in a similar way to visible light radiation depending on the nature of the substance it touches. Water vapour, CO2, and ozone, for example, effectively absorbs infrared radiation.
Thermal properties
Heat is the term for the transfer of energy. Infrared light is one of the carriers via which energy is conveyed. Infrared radiation is responsible for a large portion of the energy which reaches the planet from the sun.
Refraction
If infrared light goes from one media, such as outer space, to another medium with a different density, such as the earth’s atmosphere, it exhibits the feature of refraction, which indicates that the direction where the light is flowing suffers a minor change in direction.
Interference
This property states that if two infrared rays of the same wavelength collide, they will interfere. They will enhance or cancel each other in varying levels depending on how they meet.
Application of Infrared Radiation
Infrared radiations are used in a variety of ways, including:
Cosmetology Application:
Since infrared radiation can penetrate three to four micrometres into the skin, it is frequently used for aesthetic applications such as treating skin injuries, smoothing wrinkles, lowering the prevalence of dandruff blackheads, and so on. Its radiation also assists in the warming of the skin, resulting in improved and increased blood circulation and a constant supply of oxygen and other nutrients to the skin.
Astronomy:
In order to investigate things from space, astronomers employ infrared radiation in conjunction with optical instruments like solid state digital detectors, mirrors, and lenses. With the use of an infrared telescope, the images acquired by these devices can be deciphered.
Massage therapy
Infrared rays are employed during massage therapy to warm up the skin, which aids in muscle relaxation. It’s also preferable because they’re non-toxic and can permeate the skin.
Infrared photography
Infrared filters are commonly used to capture images during infrared photography. The photos captured for objects in the near-infrared spectrum. Infrared blockers were employed in most digital cameras to make the near infrared a pure purple white color in the final image.
Conclusion
Electromagnetic energy of various wavelengths is emitted by everything in our surroundings, such as the sun, the earth, and other entities. At the speed of visible light, electromagnetic energy moves through space in the form of sinusoidal waves. Infrared light, generally described as infrared radiation, has a wavelength of up to 1050 nm and is a form of electromagnetic radiation. It can only be seen by the human eye under special conditions as it is much higher than visible light.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






