A heat engine is a machine that transforms thermal energy into mechanical work. Internal combustion engines, jet engines and steam turbines are some examples of heat engines. Heat is used by all of these engines to produce useful mechanical work. In this article, we will explore the heat engine efficiency and questions related to heat engine efficiency according to notes on heat engine efficiency.
A heat engine
Any equipment capable of converting energy into useful work can be called a heat engine. For example, by burning coal in the air, some of its chemical energy is released. The heat generated during the combustion process can be used to heat water in a boiler and power a turbine. This, in turn, powers a generator, which produces electricity.
The conversion of chemical energy to electric energy is inefficient. As waste heat is removed from the system, there is a loss of some energy at each stage.
How does a heat engine work?
To convert heat to work, a heat engine employs a working substance, such as water or gasoline. Only when heat moves from the high-temperature reservoir to the low-temperature reservoir does mechanical energy become available for conversion to useful work. However, because some of the heat is lost to the cold reservoir, not all heat input will be converted into useful work.
A working substance, a hot reservoir and a cold reservoir are the three main components of an engine. The engine functions because of the working substance, which can be a liquid or gas. The hot reservoir receives heat input, while the cold reservoir receives heat output or excess heat.
What is a PV diagram?
PV diagrams are a primary visualisation tool for studying heat engines. Because engines typically use gas as a working substance, the ideal gas law connects the PV diagram to temperature, allowing the three basic state variables for the gas to be tracked throughout the engine cycle. Because work is only performed when the volume of the gas changes, the diagram provides a visual representation of the work performed.
The internal energy of an ideal gas varies with temperature, so the PV diagram, along with the temperatures calculated from the ideal gas law, ascertain changes in the internal energy of the gas, allowing the quantity of heat added to be determined using the first law of thermodynamics.
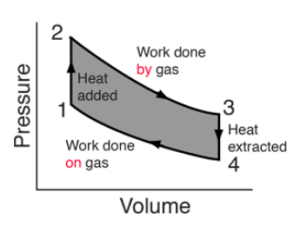
Heat engine efficiency
Heat engine efficiency is defined as the ratio of the temperature difference between the hot source and the sink to the temperature of the hot source. It is also known as the heat engine’s thermal efficiency. The total efficiency of a heat engine is feasible if the difference between hot and cold reservoirs is the greatest. There is no unit for efficiency.
The thermal efficiency of one heat engine may differ from another. Let’s look at dependable heat engines and their efficiencies to understand this better.
The following are the efficiencies of various heat engines:
- Gasoline engines in automobiles are nearly 25% efficient.
- Similarly, coal-fired power plants are only 49% efficient.
What is the heat engine efficiency formula?
Because the efficiency of a heat engine is a fraction of the heat and useful work produced, it can be expressed using a formula and a symbol. The heat energy formula’s efficiency is:
n = WQ1
In this formula,
W = Work done by the engine
Q1 = Heat coming from the source
Following each cycle, the heat engine returns to its initial stage.
∆U = 0
From the figure, we can see that:
W = Q1 – Q2
So, the heat engine efficiency is
n = Q1 – Q2/Q1
n = 1 – Q2/Q1
So, for Q = 0, efficiency will be 100%, but this is not feasible because there will be some energy loss in the system. As a result, every engine has an efficiency restriction. A reversible engine, such as a Carnot heat engine, has the highest efficiency.
Types of heat engines
Many types of heat engines work on heat engine efficiency formulas. A few of them are listed below:
Steam Engine
Steam engines run on steam generated by the combustion of coal, oil or gas. The produced steam enters the intake valve, allowing the piston in the engine to move. The engine’s movement generates useful mechanical work. The gases are released from the exhaust valve as the piston returns to its original position. After that, it is cooled into the low-temperature reservoir and the water can be reused and warmed again to begin the next cycle.
Internal combustion engine
These engines are the most widely used. The four steps of an internal combustion engine are depicted in the diagram. The gas-air mixture tends to flow into the intake valve as the piston descends. As the piston moves upward, the mixture is compressed. The spark plug kindles the compressed air, causing the gas to heat up quickly. The gas then expands, causing the piston to move downward. The burned gases are pressed out of the exhaust valve as it moves up again. As a new batch of gas-air mixture enters the cylinder, the cycle is repeated.
Conclusion
Heat engines are devices that transform thermal energy into mechanical energy. These engines operate at around 30%- 50% efficiency. This article explains the heat engine efficiency according to heat engine efficiency notes.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






