A dipole is defined as an arrangement of two opposite and equal charges linked by a line passing through their centres, with the dipole length being the distance between them. Assume that a random point exists anywhere, and we want to know how much potential the following dipole generates at that location. Because there are two charges, the electric potential is equal to the sum of the potentials produced by each of them. We’ll learn how to calculate electric potential near a dipole in this post.
Electric dipole
The electric potential is equal to the sum of the potentials produced by both charges because there are two of them. In this post, we’ll look at how to compute electric potential near a dipole. Now, in order to obtain our formula, we must imagine the point P to be far away from the charges, and the dipole length to be considerably shorter than the P’s distance. Also, because the distance between the locations changes, the potential across them will be variable. As we all know, potential and distance are inextricably linked.
The symbol for an electric dipole is.
An electric dipole is defined as the product of the magnitude of the charges multiplied by the distance between them, and it may be expressed mathematically as follows:
An electric dipole’s magnitude is stated as:
P=Qd
Electric Dipole Moment Direction
The electric dipole moment is a vector quantity with a definite direction of travel from negative to positive charge. It’s important to remember, though, that this orientation standard is only used in Physics. The polarity of a substance should be reversed in chemistry, from positive to negative. The line that travels along the direction of an electric dipole is known as the dipole’s axis.
Due to a Dipole, there is an electric potential (V)
Assume that a dipole is generated by two charges separated by d, –q at A and +q at B. Assume that O is the AB’s midpoint. The electric potential owing to a dipole at every point P where OP = r is:
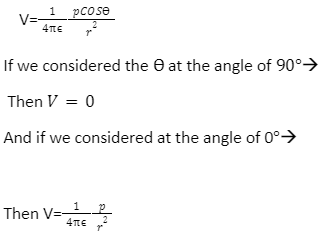
What does having an electric dipole moment imply?
It’s essentially a precise measurement of an electric dipole’s strength. According to mathematics and scientific studies, the dipole moment magnitude is the product of either charge and the separation distance ‘d’ between them. Keep in mind that the dipole moment is a vector measurement with a charge direction of negative to positive.
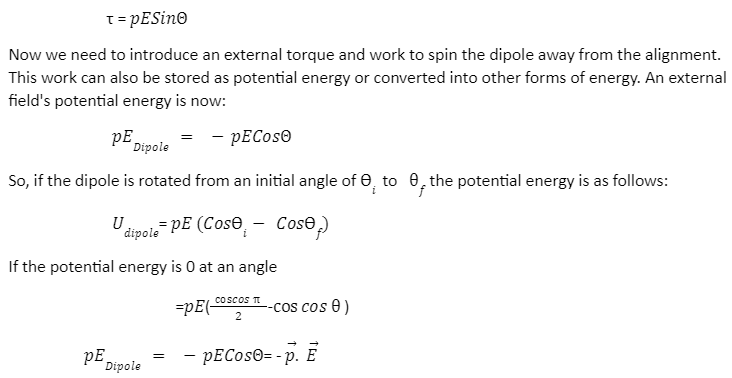
A Dipole’s Electric Potential Energy
We know that equal and opposite forces cancel each other out in a dipole, but the torque acting parallel to the system rotates it.
If p denotes a dipole moment that is not aligned with the uniform electric field E, then, therefore, torque is responsible for aligning E to p, and its magnitude is:
Dipole’s Physical Importance
The electric dipole is important in both electrostatics and chemistry. The distance between two charges is zero because the centres of positive and negative charges in most molecules are in the same place The zero-dipole moment category includes carbon dioxide and methane. This type of molecule is known as a nonpolar molecule. Because the centres of positive and negative charge do not coincide, polar molecules exhibit a persistent dipole moment. A polar molecule is one in which the centre of mass of the positive charge does not match to the centre of mass of the negative charge.
Non-Polar molecules are those in which the positive charge’s centre of mass is the same as the negative charge’s centre of mass.
Polar compounds have permanent dipole moments. These dipoles are oriented randomly in the absence of an external electric field. The polar molecules align themselves in the direction of the electric field when it is applied. If a system’s net charge is zero, that doesn’t mean there won’t be an electric field or that it won’t be there. This was demonstrated using the electric dipole moment. As a result, learning how to investigate an electric dipole is essential. Understanding polarisation aids our understanding of dipoles and dipole moments.
Conclusion
The electric potential is equal to the sum of the potentials produced by both charges because there are two of them. In this post, we’ll look at how to compute electric potential near a dipole. A dipole is defined as an arrangement of two opposite and equal charges linked by a line passing through their centres, with the dipole length being the distance between them. The numerical and direct derivations are heavily weighted in the Physic chapter, so students are recommended not to skip any key topics. The exam asks additional questions about derivations or topics like energy stored in capacitors, potential owing to electric dipole, and potential energy of a system of charges. The exam includes both Short Answer and Long Answer questions about numbers. Because complicated capacitor combinations will be tested in the exam to determine effective capacitance or charge on plates, all students are encouraged to study the material thoroughly.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






