The carbon atom undergoes sp³ hybridisation when it is exciting. But the synthesis of the methane molecule results in four half-filled sp³ hybrid orbitals aligned in tetrahedral symmetry in the space surrounding the carbon atom. Each of these sp³ hybrid orbitals creates an sp³-s bond with one hydrogen atom. As a result, four hydrogen atoms form four σ bonds with carbon. Here we know how this hybridisation is done and what are requirements to complete the hybridisation process? This article explains how methane is hybridised and explains more about the hybridisation of methane.
What is hybridisation?
According to the hybridisation of methane notes, hybridisation is the process of merging two atomic orbitals to form a new type of hybridised orbitals. Due to the intermingling of atoms, these hybrid orbitals possess different energies, geometries, etc.
Atomic orbitals that exhibit the same energy level are primarily responsible for hybridisation. If their energies are equal, both filled and half-filled orbitals can participate in the hybridisation process if their energies are equal. This process can also be called the extension of valence bonds theory, which helps understand the bond formation, bond energy, and bond lengths.
The new orbitals formed due to this process are called hybrid orbitals. These orbitals also define molecular geometry and different atomic bonding features.
For example, the s – orbital of the carbon valence-shell joins with three valence-shell of p orbitals to produce four single bonds. Therefore, this combination helps in producing four sp³ mixes. If the carbon is bonded with four distinct atoms, it will be organised in a tetrahedral arrangement.
Salient features of hybridisation
- hybridisation occurs when the energies of atomic orbitals are equal.
- The shape of a molecule can be predicted if the hybridisation of the molecule is known beforehand.
- The number of orbitals generated will be equal to the number of atomic orbitals.
- Hybridisation does not happen in a single gaseous atom; instead, it occurs during bond formation.
- Hybridisation does not necessarily involve the participation of all half-filled orbitals. The orbitals that are filled but have little energy can also participate in this process.
- The hybrid orbital’s larger lobe is always positive. At the same time, the smaller lobe on the opposite side is always negative.
Different shapes of hybridisation
- Linear: The interaction of two-electron groups results in sp hybridisation with an orbital angle of 180°.
- Trigonal planar: The trigonal planar hybridisation is formed by three electrons, resulting in sp² hybridisation, which has orbitals of 120° apart.
- Tetrahedral: Tetrahedral hybridisation has sp³ hybridisation with a 109.5° orbital angle. In this hybridisation, four-electron groups are involved.
- Trigonal bipyramidal: The five electron groups result in sp³d hybridisation with orbital angles of 90° and 120°.
- Octahedral: Octahedral hybridisation is the result of six electron groups that lead to sp³d² hybridisation, and the orbitals are 90° apart.
Rules for Identifying the hybridisation Type
The following rules must be followed to determine the type of hybridisation in a chemical or an ion.
- Find the total number of valence electrons
- Calculate the number of duplex or octet duplex
- Find lone pair electrons
The number of orbitals = Number of duplex or octet + Number of lone pair electrons
- In case there are no lone pairs of electrons, the orbitals and molecules will have different geometry.
sp3 hybridisation of Methane
Valence bond theory uses a concept called orbital hybridisation. For example, four Valence orbitals of carbon (one 2s and three 2p orbitals) combine (keep in mind: orbitals are defined by equations) to produce four equivalent hybrid orbitals. These are known as sp³ orbitals as they are made up of ones and three p orbitals. Each of the four valence electrons on the carbon now becomes a single sp³ orbital. As a result, four unpaired electrons form in the new electron configuration.
sp³ orbitals are combinations of s and p atomic orbitals. An electron stays in each sp³-hybridised orbital, and these electrons do not attract each other.
The four sp³-hybridised orbitals circle the carbon nucleus as far away as possible to minimise their electron repulsion. It results in the tetrahedral structure predicted by VSPER. An “sp³-hybridised carbon atom” is the carbon atom that is present in methane.
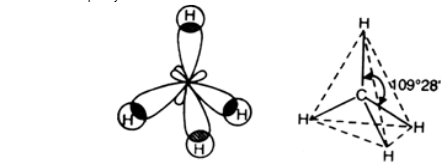
The sp³ hybrids have bigger lobes in the four corners of a tetrahedron which forms the angle of 109.28o between any two orbitals.
Conclusion
Here, we have learned all about the hybridisation of methane, how methane is hybridised, and what happens in methane hybridisation. When it comes to methane hybridisation, the core carbon atom is sp³ hybridised. In the valence shell of carbon, one 2s orbital and three 2p orbitals combine to form four sp³ hybrid orbitals of equal energy and shape.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out



