Introduction:
In a molecule, two atomic orbitals come together to make a hybrid orbital. Hybridisation is the term used for this procedure. The atomic orbitals with similar energies are combined throughout the hybridisation procedure, which usually involves mixing two orbitals or two ‘p’ orbitals or combining an ‘s’ orbital with a ‘p’ orbital and an ‘s’ orbital with a ‘d’ orbital. Hybrid orbitals are one of the novel orbitals that result from this process. Hybrid orbitals are particularly valuable in describing atomic bonding characteristics and molecule’s geometry.
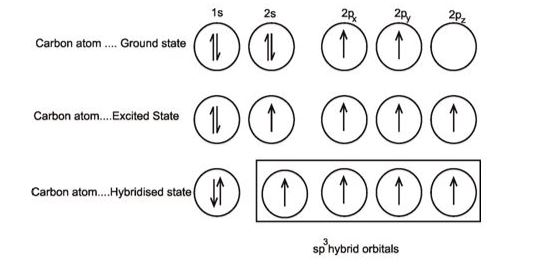
Let’s take the example of a carbon atom. The valence-shell s orbital mixes along three valence-shell p orbitals to generate four single bonds. This combination produces four sp3 hybridisation mixes that are comparable. These will have a tetrahedral structure from the carbon bound to four distinct atoms.
Hybridisation’s key characteristics
The following are the key characteristics of hybridisation:
- The number of hybrid orbitals is the same as that of hybridised atomic orbitals.
- The energy and geometry of the hybridised orbitals are always the same.
- Pure atomic orbitals are less successful than hybrid orbitals at forming stable bonds.
- These hybrid orbitals are oriented in space in the desired direction to minimise electron pair repulsions and maintain a stable configuration. As a result, the kind of hybridisation exhibited determines the molecule’s shape.
Hybridisation requires certain circumstances
(i) The orbitals in the atom’s valence shell become hybridised.
(ii) The energy of the orbitals undergoing hybridisation has to be identical.
(iii) There is no need for electron promotion before hybridisation.
(iv) Only half-filled orbitals do not have to participate in hybridisation. Hybridisation can involve even full valence shell orbitals in rare circumstances.
Types of hybridisation
Hybridisation involving s, p, and d orbitals can take several forms. The following are the many forms of hybridisation:
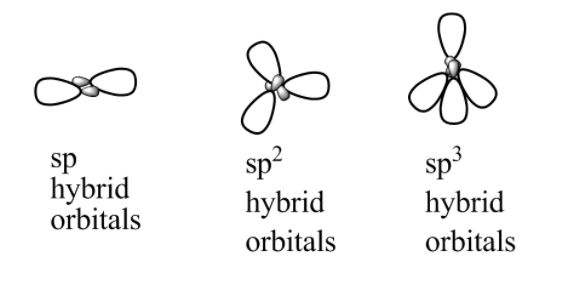
(I) sp hybridisation: The mixing of one s and one p orbital produces two equivalent sp hybrid orbitals in this kind of hybridisation. If the hybrid orbitals lie along the z-axis, the orbitals s and pz are acceptable for sp hybridisation. Each sp hybrid orbital has 50% s-character and 50% p-character. A molecule with linear geometry has an sp-hybridised core atom and is connected straight to two additional central atoms. The term “diagonal hybridisation” refers to this sort of hybridisation.
With projecting positive lobes and extremely tiny negative lobes, the two sp hybrids point in opposing directions along the z-axis, allowing for more efficient overlapping and the development of stronger connections.
(II) sp2 hybridization: One s and two p-orbitals are involved in this hybridization, resulting in three equivalent sp2 hybridised orbitals. The electronic configuration of the central boron atom in the BCl3 molecule, for example, is 1s22s22p1. One of the 2s electrons is promoted to the unoccupied 2p orbital in the excited state, leaving the boron with three unpaired electrons.
Three sp2 hybrid orbitals result from the hybridisation. The resulting three hybrid orbitals overlap with a trigonal planar arrangement. Chlorine’s 2p orbitals combine to generate three BCl molecule bonds. With a Cl-B-Cl link, the geometry is trigonal planar at a 120° angle.
(III) sp3 hybridisation: This sort of hybridisation may be discussed using the CH4 molecule as an example, where one s-orbital and three p-orbitals of the valence shell are mixed to generate four sp3 hybrid orbitals with equivalent energies and shapes. Each sp3 hybrid orbital has 25 percent s-character and 75 percent p-character. The four sp3 hybrid orbitals that result are pointed towards the tetrahedron’s four corners. The angle between each sp3 hybrid orbital is 109.5°.
Other examples of sp3, sp2, and sp hybridisation
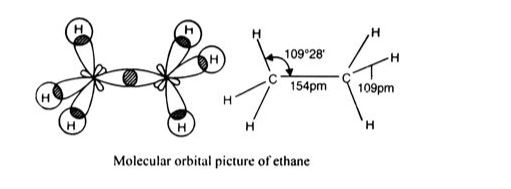
sp3 hybridisation in C2H6 molecule: Both carbon atoms in the ethane molecule are in an sp3 hybrid form. One of the four sp3 hybrid orbitals of each carbon atom overlaps axially with comparable orbitals of the other atom to create an sp3-sp3 sigma bond, while the other three hybrid orbitals of each carbon atom are utilised to generate sp3 –s sigma bonds with hydrogen atoms.
As a result, the C–C bond length is 154 pm, whereas each C–H bond length is 109 pm.
In constructing the ethene molecule, one of the carbon atom’s sp2 hybrid orbitals crosses axially with another carbon atom’s sp2 hybridised orbital to create the C–C sigma bond. The carbon-carbon link in the ethene molecule comprises one sp2–sp2 sigma bond and one pi bond between p orbitals that are not utilised in hybridisation and are perpendicular to the molecule’s plane; the bond length is 134 pm. The C–H bond has a length of 108 pm and is sp2–s sigma. The H–C–H bond angle is 117.6 degrees, while the H–C–C bond angle is 121 degrees.
Conclusion
When two atomic orbitals merge to form a hybrid orbital in an isolated molecule, the redistribution of orbital energy of particular atoms happens, giving orbitals the equivalent energy. Hybridisation is the term for this procedure. The new orbitals created due to this process are known as hybrid orbitals. Linus Pauling established the notion of atomic orbital hybridisation to explain the distinctive forms of polyatomic compounds. The projection and geometrical shapes of molecules like BeCl2, BCl3, CH4, NH3, and H2O are explained using sp, sp2, and sp3 hybridisation of atomic orbitals of Be, B, C, N, and O.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




