Werner, a well-known scientist, proposed his theory of coordination compounds in 1823, which describes the formation and structure of complex compounds and was later named Werner’s Theory of Coordinate Compounds.
He was given the Nobel Prize for this theory, and he is also known as the “Father of Coordination Chemistry.”
Werner’s theory’s important postulates are as follows:
- The central metal or metal atoms in coordination compounds have two forms of valency which are primary valency and secondary valency. The oxidation state corresponds to the primary valency, while the coordinate number corresponds to the secondary valency.
- Each metal atom has a fixed number of secondary valencies, like it has a fixed coordinate number.
- The metal atom satisfies both its primary and secondary valencies. Negative ions satisfy primary valencies, but neutral molecules or negative ions satisfy secondary valencies.
- The secondary valencies are always directed towards a particular place in space, resulting in determination of geometry of the coordinate compound’s. As an example: A metal ion’s secondary valencies are grouped octahedrally around the central metal ion if it has six of them. If the metal ion contains four secondary valencies, they are organized in a tetrahedral or square planar pattern around the center metal ion. The stereochemistry of the complex ion is thus determined by the secondary valency while the primary valency is non-directional.
Examples Based to explain Werner’s Theory Postulates
The following are the structures of various cobalt amines based on Werner’s theory of coordination compounds
Cobalt has a primary valency of three and a secondary valency (coordination number) of six. Secondary valencies are depicted by thick lines, while primary valencies are represented by broken lines.
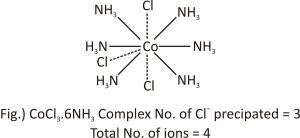
CoCl3.6NH3 Complex: The coordination number of Co in this compound is 6, and all six secondary valencies are met by ammonia molecules . Cl- ions satisfy the three primary valencies . These have a non-directional character. When silver nitrate is added, these chloride ions precipitate instantly. In this scenario, there are four ions: three chloride ions and one complex ion. While writing the compound’s formula, the central ion and neutral molecules or ions satisfying secondary valencies are enclosed in square brackets. Therefore, the complex can be written as [Co(NH3 )6]Cl3 and is depicted in fig.
CoCl3.5NH3 complex: The coordination number of cobalt in this compound is also 6, but the number of NH3 molecules is reduced to 5 from 6 and one remaining slot is now occupied by chloride ions. Because it has both primary and secondary valency, this chloride ion exhibits dual behavior. In the figure, the secondary valency is represented by a full line, while the main valency is represented by a dotted line.
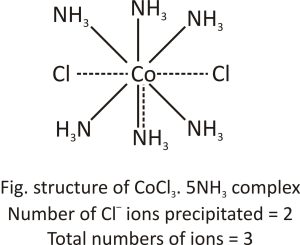
This structure satisfies cobalt’s three primary and six secondary valencies. As a result, the complex formed can be written as [CoCl(NH3)5]Cl2 with five ammonia molecules and one chloride ion inside the square brackets and two chloride ions outside the brackets.
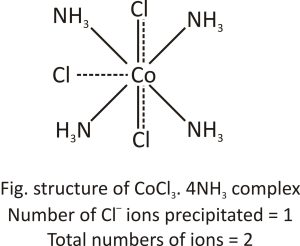
CoCl3.4NH3 complex: Two chloride ions in this compound exhibit dual behavior, satisfying both Primary and Secondary Valencies. This compound will precipitate AgNO3, which corresponds to one Cl- ion, and the total number of ions, in this case is two. As a result, it can be written as [CoCl2(NH3)4]Cl.
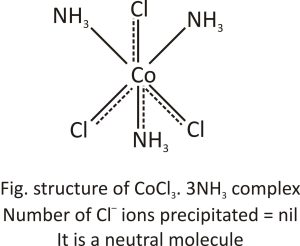
CoCl3.3NH3 complex: In this molecule, three chloride ions satisfy primary and also secondary valency. At room temperature, silver nitrate does not precipitate Cl-. Hence, the complex compound behaves as a neutral non-conducting molecule. It may be written as [CoCl3(NH3)3].
isomers in coordination compounds and Werner’s Theory
Werner shifted his attention to the geometrical configurations of the coordinated groups around the central cation and effectively explained the origin of these compounds’ optical and geometrical isomerism. The following are some examples:
[CoCl2(NH3)4]Cl: Werner stated that there are three theoretical structures for this complex. Planar, trigonal prisms and octahedral are examples. There are three possible isomers for a planar structure, three for a trigonal prism, and two for an octahedral structure.
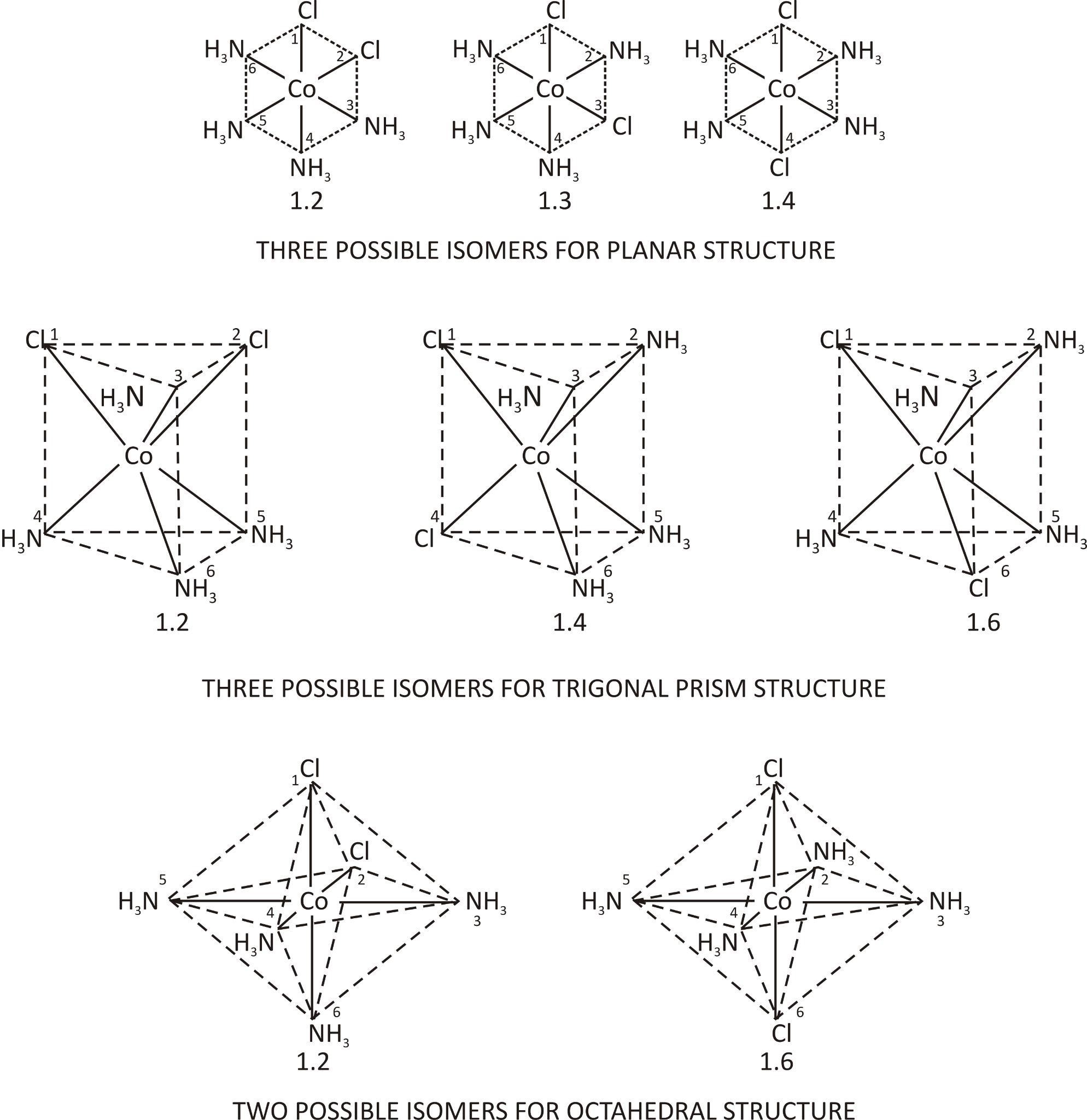
Werner concluded that the geometrical arrangement of the coordinated group around the central atom in this compound was octahedral since only two isomers of the compound could be isolated. Werner was able to conclude that the six coordinated complexes have octahedral geometry in the case of various additional complexes in which the coordination number of the central atom was six.
He examined the geometry of complexes with a central metal atom and a coordination number of 4. He presented two possibilities for the structure. Tetrahedral and square planar.
Complex [PtCl2(NH3)2]: The coordination number of the metal in this complex is 4, and Werner discovered that it existed in two isomeric forms, cis and trans. This demonstrates that all four ligands are on the same plane. As a result, the structure should be square planar or tetrahedral.

Limitations of Werner’s Theory:
Werner’s Theory was not free from limitations. The common drawbacks of the theory are:
- It was not enough to explain the inability of all elements to form coordination compounds.
- The Werners theory could not explain the directional features of bonds in numerous coordination compounds.
- It does not explain the color, the magnetic and optical properties demonstrated by coordination compounds.
Conclusion
Werner shifted his attention to the geometrical configurations of the coordinated groups. The central metal or metal atoms in coordination compounds have two forms of valency.The metal atom satisfies both its primary and secondary valencies.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





