Mesomeric Effects are an essential topic in organic chemistry. Students preparing for the IIT JEE examination must know about the mesomeric effect. The terms mesomeric effect, mesomerism, and mesomer were coined by scientist Ingold in 1938. Mesomerism is a term that was coined by scientist Linus Pauling and is synonymous with resonance. However, in English, the term “resonance” has grown in popularity and is now extensively used. This article will discuss the meaning and types of mesomeric effects notes.
Meaning of Mesomeric Effects
In chemistry, the mesomeric effect is a property of substituents or functional groups in a molecule. The mesomeric effect (M) causes an electron excess or deficit depending on the type of the substituents as a result of interaction through the -electrons. The electron density and, as a result, the chemical shift will alter if a substituent with a double bond or nonbonding electrons is directly linked to a conjugated system.
The effect, which is symbolised by the letter M, is used qualitatively to explain the electron-withdrawing or releasing properties of substituents based on relevant resonance structures. When a substituent is an electron-withdrawing group, the mesomeric effect is negative (–M), and when a substituent is an electron-donating group, the effect is positive (+M).
Types of Mesomeric Effects
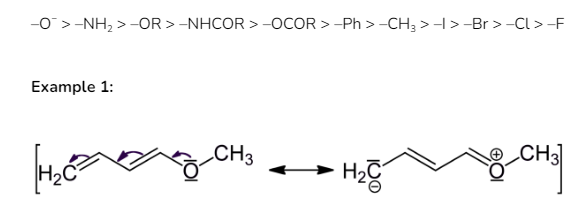
+M EFFECT
The (+M) effect or positive mesomeric effect occurs when electrons or pi electrons are moved from a specific group to a conjugate system, thereby boosting the electron density of the conjugated system.
Furthermore, it is pertinent to note that the group must have either a lone pair of electrons or a negative charge to produce the +M effect.
The +M effect causes the conjugate system to have a negative charge or the electron density to increase on the conjugate system. These conjugate complexes have a higher electrophile reactivity and a lower nucleophile reactivity.
The positive mesomeric effect can be seen in the following group in this particular order:
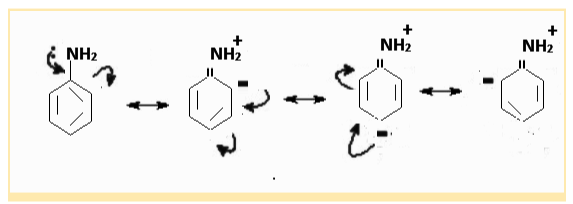
Example 2: In aniline, the -NH2 group likewise has a +R impact. Through delocalisation, it releases electrons towards the benzene ring. The electron density on the benzene ring increases as a result, especially at the ortho and para locations. As a result, aniline activates the ring, allowing it to undergo electrophilic substitution.
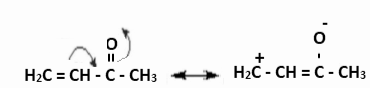
-M EFFECT:
The negative mesomeric (–M) effect occurs when pi-bond electrons are moved from the conjugate system to a specific group, resulting in a drop in the conjugate system’s electron density.
Furthermore, it is pertinent to note that the group must have either a positive charge or a vacant orbital for the –M effect to occur.
The –M effect makes a molecule more reactive to a nucleophile by lowering the electron density in the conjugate system but also makes it less reactive to an electrophile for the same reasons.
The negative mesomeric effect can be seen in the following group in this particular order:
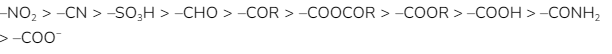
Example 1: The carbonyl group’s negative resonance effect (-R or -M) is depicted below. It delocalised electrons, thus removing them and lowering electron density, especially on the third carbon.
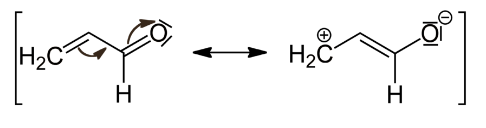
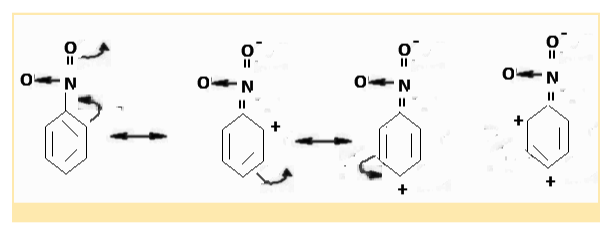
Example 2: Due to delocalisation of conjugated electrons, the nitro group, -NO2, in nitrobenzene exhibits the -M effect, as seen below. The electron density on the benzene ring is reduced, especially in the ortho and para locations.
Applications Of Mesomeric Effect
The various application of the mesomeric effect are as follows:
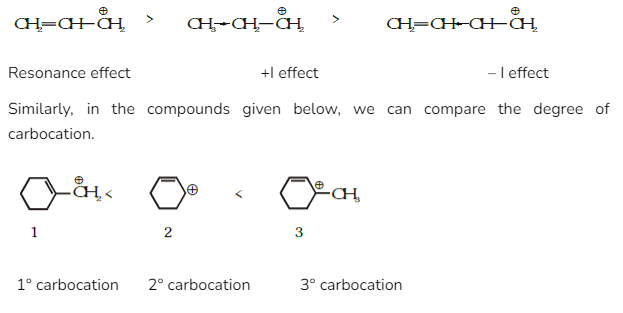
1.Carbocation Stability
Carbocation’s stability is improved by resonance, which is one of the uses of the mesomeric effect. Due to the impact of resonance, all aromatic compounds are always more stable than non-aromatic compounds.
For example, in the compounds given below, we can compare the stability order of each combination.
2.Stability of Carbanion
The mesomeric effect or resonance increases the Carbanion’s stability
For example, we can compare the stability order of the following compounds:
In these compounds, the mesomeric effect affects the stability of the carbanion in a manner that the order of stability is I > II > III.
3.Stability of Free Radicals
The stability of free radicals is increased by the mesomeric effect or resonance.
For example, in the following compounds,
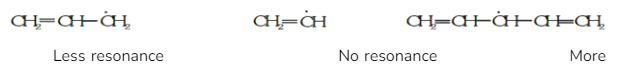
resonance
Thus the stability order of the compounds is III > I > II
Acidic and Basic Strength
Acidic strength:
- The -M impact is directly proportional to the acidic intensity.
- The -I impact is directly proportional to the acidic intensity.
- The +M action is indirectly proportional to acidic strength.
- The +I effect is inversely proportional to acidic strength.
Thus, this can be represented as:
Acidic strength ∝ -M effect ∝ -I effect ∝ 1 / +M ∝ 1/+1
Basic strength:
- The -M effect is directly proportional to the degree of acidity.
- The -I effect is directly proportional to the acidity of the environment.
- The acidic strength is indirectly related to the +M action.
- The acidic strength has an inverse relationship with the +I effect.
Thus, this can be represented as:
Basic strength ∝ +M effect ∝ +I effect ∝ 1 / -M ∝ 1 / -1.
Conclusion
Thus through the types of mesomeric effects notes, we have learnt the meaning and types of mesomeric effects. We now know that as a result of interaction through the -electrons, the mesomeric effect (M) generates an electron surplus or deficit depending on the type of substituents. If a substituent with a double bond or nonbonding electrons is directly attached to a conjugated system, the electron density and, as a result, the chemical shift will change. Further, there are two types of mesomeric effects: +M effect and -M effect according to types of mesomeric effects notes.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




