Types of Ligands
Coordination compounds consist of a metal ion that is covalently bonded to ligands. Ligands are charged or neutral molecules or ions that can form coordination bonds with the transition metal. The word “ligand” comes from the Latin word Ligandus which means “to bind”. They donate electron pairs to the empty hybridised orbital of the central metal.
There are various types of ligands. They can be classified based on their denticity, complex molecular structure, chemical nature, and field strength.
Denticity
Denticity is defined as the number of donor groups that are involved in the process of bonding with metal. Ligands can be classified based on their denticity as follows:
- Unidentate Ligand: If a ligand has only one donor site/group, it is called unidentate. They are also known as ‘one-toothed’, as they attack (or bite) the metal only at only one place. Examples of unidentate ligands include Cl-, NH3, etc. They donate one lone pair of electrons to the metal.
- Multidentate Ligand: If a ligand has more than one donor site/group, it is called multidentate. It can further be divided into the following types based on the number of donor sites:
- Bidentate ligands: They have two donor sites, and both of them can participate in the bond formation simultaneously. Ethylenediamine is an example of a bidentate ligand in which two nitrogen atoms can donate electrons simultaneously to the metal centre.
- Tridentate and tetradentate ligands: They have three and four donor sites respectively. Diethylenetriamine is a tridentate ligand having three nitrogens that can donate electrons. Triethylenetetrammine is a tetradentate ligand having four nitrogens.
- Hexadentate ligands: They have six donor sites. Example: EDTA
Polydentate Ligand: When a ligand can exhibit multiple denticities, it is called polydentate. EDTA (ethylenediaminetetraacetate) is an example that can donate different numbers of electron pairs, ranging from one to six. It can bind with the metal centre via two nitrogens or four acetate groups’ Oxygen. The actual denticity depends on external factors like pH.
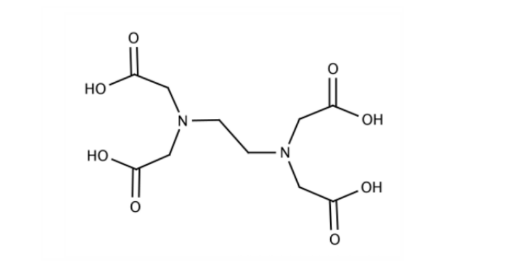
- Ambidentate Ligand: A ligand that has more than one donor site but only one of them can be involved in bonding at one time. For example, SCN- is an ambidentate ligand as it can donate from both sulphur and nitrogen centres. NO2- is ambidentate as it can donate from both oxygen and nitrogen centres.
Chelate effect in ligands
Multidentate ligands exhibit the chelate effect as they can bind with the central metal through more than one donor site simultaneously. The central metal ions exhibit greater affinity for ligands that show the chelating effect as a ring-like structure forms around the metal, which provides better structural stability.
Examples of chelating ligands include EDTA and Diethylenetriamine.
Bridging Ligands
Ligands can act as a bridge between two metal ions. Carbonyl and hydride act as bridging ligands in a few compounds.
In Carbonyl bridging in Fe2(CO)9, out of 9 carbonyl ligands, three of them act as a bridge.
Types of ligands based on charge
Based on the charge, the following are a few types of ligands:
- Anionic Ligands: They have a negative charge. Examples include chloride, hydride, and cyanide.
- Cationic Ligands: They have a positive charge. Their compounds are rather rare. Ligands containing pyridine come under this category.
- Neutral Ligands: They do not have a charge. They have a lone pair of electrons in their orbitals. Examples include water, ammonia, and ethylenediamine.
Spectrochemical series for Ligands
Ligands can be arranged into a spectrochemical series based on their ability to split the d-orbitals of the metal. The ligands that split the d-orbital into two types of orbitals having a greater energy difference are called Strong Field Ligands. The ligands that split the d-orbital into two types of orbitals having a smaller energy difference are called Weak Field Ligands.
I- < Br- < Cl- < NO3- < F- < OH- < H2O < Pyridine < NH3 < NO2- < CN- < CO
Strong Field Ligands can cause electrons to pair up in the d-orbital and give low-spin and diamagnetic complexes.
Conclusion
Denticity is an important concept when it comes to ligands. Several types of ligands examples are there for different denticities. Multidentate ligands can show the chelate effect that helps stabilise the complex to a large degree. Ligands can also act as a bridge between two metal ions.
Ligands can be classified based on their charge as well. An important classification is concerning their field strength, which ultimately determines the hybridisation and geometry of the complex molecule.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





