In the field of chemistry, the pH of any substance plays an important role. One must identify and know the nature of the substance, solution, or liquid, including knowledge of the acidic, basic, or neutral nature of any chemical substance or solution. This knowledge plays a vital role in our day-to-day life.
If the acidic content in the food one ingests is above the normal limit, it can harm the human body, including causing a fatality. This module will discuss all the basics an individual needs to be aware of, including the concept, the significance, the uses, and the limitations of the Henderson-Hasselbalch equation.
History of the Henderson equation
Lawrence Joseph Henderson developed an equation for calculating the pH of a buffer solution in 1908. In 1917, Karl Albert Hasselbalch reformulated this equation in logarithmic terms, which is now frequently used in chemistry.
What is the Henderson equation?
To determine the pH of a buffered solution, an individual must utilise the Henderson-Hasselbalch equation. This equation is derived from the kinetics of reversible reactions, which describe the characteristics of weak acids and bases in a given solution.
The Henderson-Hasselbalch equation gives the reader an interaction between the acid dissociation constant (pKa) and the pH of acids (in aqueous solutions). In simple words, the Henderson equation is a chemical equation that relates the acid dissociation constant to the pH of the aqueous solution of an acid.
This equation is often used in chemistry to calculate and find out the isoelectric point of various proteins. The isoelectric point of a protein is the pH at which its net charge becomes zero. In other words, the protein does not accept or donate protons.
The limitations of the Henderson-Hasselbalch equation:
- The Henderson-Hasselbalch equation does not account for water’s self-dissociation; it cannot provide the correct pH values for highly diluted buffer solutions.
- As Henderson’s equation implies that the concentration of the conjugate base of acid at chemical equilibrium will stay the same as the nominal concentration, the Henderson-Hasselbalch equation falls short of predicting an appropriate result for strong acids and strong bases, which means that the binding of protons to the base is neglected.
- The assumption stated in the Henderson equation might not be applicable when associating with strong bases or acids.
- The most important assumption in this equation is that the concentrations of acid and conjugate bases always stay constant during the equilibrium.
- The relevance of water hydrolysis and its influence on the pH of the entire solution is often overlooked. Likewise, the hydrolysis of an acid and base dissociation are overlooked.
Acid dissociation constant
The acid dissociation constant ‘Ka’ quantitatively measures the strength of an acidic solution. Ka represents the equilibrium constant for any given dissociation reaction of an acid in an aqueous solution.
HA (aqueous) ⇌ H+(aqueous) + A−(aqueous)
In the above reaction:
HA = the Generic Acid
A- = the Conjugate base of a given acid
H+ = is the Hydrogen proton or ion
These quantities in the equation remain in equilibrium even if their concentrations do not show any change with time.
The value of Ka, like other equilibrium constants, is determined with the help of the concentrations measured in terms of moles per litres of each aqueous component at the state of equilibrium. The following is the expression for the acid dissociation constant (Ka):
Ka=[H+][A-]/[HA]
The Henderson equation
There are various ways of writing the Henderson equation. Two of the most frequently used formulae/equations are:
pH = pKa + log [conjugate acid]/[weak acid]
pOH = pKb + log [conjugate acid]/[weak base]
The uses and significance of Henderson equation
The main objectives of the Henderson-Hasselbalch equation in the field of chemistry includes:
- To find the state of protonation of various biomolecule functional groups in a buffer of pH 7.
- To determine how exactly the relative strengths of the conjugate base of an acid can be examined by using the values of Kb and Ka for the bases and acids, respectively.
- To determine if a given aqueous solution of salt will either be basic, acidic, or neutral in nature, depending on the specified values of Ka and Kb for a conjugate pair of acids and bases.
- To explain how a buffer solution, basic or acidic, can withstand a substantial pH change when little volumes of acid or base are introduced to the buffer solution.
- It is used to describe and know the buffer solution in chemistry.
- It is used to determine and know the buffer capacity in chemistry.
- It is used to know how a basic or an acidic buffer solution in chemistry is prepared.
- To calculate the pOH/ pH, ions in a solution containing a base given the initial concentration of the acid-base.
Conclusion
This article tells an individual about the concept, uses, significance, and limitations of the Henderson-Hasselbalch equation. The Henderson-Hasselbalch equation helps an individual interested in the discipline of chemistry gain an understanding of how to measure a buffer solution and examine it. Being well-versed with the concept of the Henderson-Hasselbalch equation will be useful for future chemists and chemical engineers.
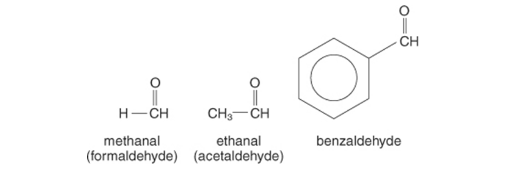
Nomenclature
The standard method or perhaps the IUPAC system is used to name aldehydes. The familiar words of aldehydes have been obtained from the carboxylic acid’s common ones. Consider the following scenario:
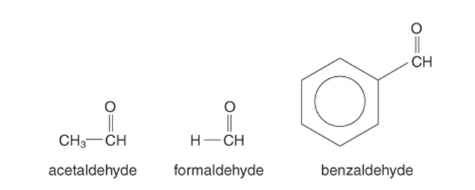
The IUPAC system follows a set of rules to create compound identities. Basic rules for naming aliphatic aldehydes are as follows:
- Locate the carbonyl group inside the longest-running chain of carbon atoms.
- The alkane name with the same amount of carbons is used as the parent name.
- Remove thee from the alkane title and replace it with al.
- Assign the lowest possible number to the carbonyl carbon, mainly in the chain.
- Identify and name substitutes.
The following are some examples of IUPAC naming:

The term carbaldehyde is added to the designation of the ring structure in the IUPAC system to identify cyclic, aliphatic, & aromatic aldehydes.
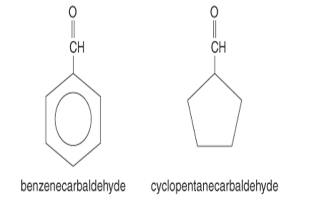
Rather than benzenecarbaldehyde, the majority of chemists call it benzaldehyde. This alternative term is also used in many sources, such as articles.
Chemicals Formula
RCHO is the chemical formula for such an aldehyde. R stands for a hydrogen ion or a carbon/hydrogen string, CO again for carbonyl and H for the hydrogen connected to the carbonyl sequence in this formula. It’s crucial to state the equation in this order because it builds the link between the aldehydic hydrogen and the carbonyl.
Aldehyde physical characteristics
Aldehydes belong to organic chemical compounds whose general balanced equation is R-CHO. R can be hydrogen or a substituted or unsubstituted hydrocarbon revolutionary.
Several aldehydes seem to be unpredictable, flammable liquids producing vapour in explosive quantities at room temperature. Lesser aldehyde components, as well as those with an unsaturated and replaced chain, need the most stringent fire and explosion prevention measures, as well as the most extensive irritating property precautions.
Aldehyde Applications
- Formaldehyde is utilised as a cleanser and organic specimen preservation.
- Glasses are silvered with an aldehyde.
- Formaldehyde is a chemical used to make a wide range of polymers and resins.
- Fragrance and the dyes (colour) business both utilise benzaldehyde.
Aldehyde Applications
1.Antioxidant
Formalin is a chemical that is used to preserve biological and anatomical specimens. This is a popular method of specimen storage at major research institutes. It is used to clean medical instruments.
2.Producing polymeric goods
Have you ever wondered why germicides, insecticides, & fungicides, among other polymeric goods, are being used to keep bacteria, bugs, and fungus at bay? This is due to the formaldehyde element employed in manufacturing these items.
3.Antiseptic
Due to its powerful ingredients, it reacts to cleanse water. Tissues are also hardened by formalin.
4.Paper-making
Acrolein is a necessary component in the paper industry since it regulates its slime.
5.Bacterial Elimination
This compound kills bacteria found in oil wells & chilled water tanks.
6.Preservative
Acetaldehyde is a flavouring ingredient and a preservative for fruits and seafood.
7.Rubber & Tire Manufacturing
Solvents containing benzene are appropriate to be used in the rubber production process.
8.Printing/painting
Because benzene is included in base & overcoat paints, it is commonly employed in the printing industry to repair and sanitise printing equipment.
9.Chemicals/Plastics
Benzyne is a chemical used to create plastics and synthetic materials such as nylon. Benzene is used in several ways in the plastics industry, such as cleansers.
10.Petroleum/Oil/Asphalt
Aldehyde is a chemical that is used to make petroleum products like gasoline. It is used to produce asphalt, which roofing firms utilise.
Conclusion
Aldehydes seem to be organic molecules with a carbon atom at the end. Because CHO is one of their main components, these are classified as compounds that contain CHO. Dehydrogenation of alcohol removes the hydrogen ion from the oxygen molecule, resulting in aldehydes. The carbon subsequently forms a double bond as a result of the reaction. Aldehydes are commonly utilised in industry to manufacture other chemicals. Aldehydes are used in various methods, many prevalent in a wide range of industries. Aldehydes are classified into four categories. Acrolein, Acetaldehyde, and Benzaldehyde are all forms of formaldehyde, which are carbon molecules, oxygen, and carbon.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





