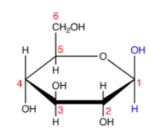
The Haworth representation of glucose structure gives the three-dimensional perspective of the molecular structure of the glucose compounds. To represent the structure and its composition, many methods of projections are used. However, Fischer’s projection and Haworth’s projection are most widely used.
In the following article, we will discuss the nature of carbohydrates and glucose compounds. We will also look into the Haworth representation of glucose structure examples; the article will compose a comprehensive note for the Howard representation of Glucose structure’s importance.
Carbohydrates
Carbohydrates are compounds widely found in organic matters; plants majorly produce carbohydrates. Sugar (cane sugar), glucose, and starch are common examples of carbohydrates. They also form a large group of organic compounds. Most of the carbohydrate compounds follow the following general formula,
Cx (H2O)y
In the early years, carbohydrates used to be considered hydrates of carbon compounds; hence the name carbohydrate was given to them. But, this formula may also give nomenclature for non-carbohydrate compounds. For example, acetic acid (CH3COOH) also follows this formula, C2.(H2O)2, but it is an acid.
Carbohydrates can be defined as active polyhydroxy aldehydes or ketones based on their chemical composition and concentrations. Some of the carbohydrates are called sugars and are called glucose.
Projection of Structure of Compounds
In studying chemical and biochemical compounds and their structure, we often see several different methods for presenting molecules and their chemical structure in three dimensions. However, most organic chemists prefer to use the dashed/solid wedge method to show stereochemistry; other scientists often use methods called Fischer projections and Haworth projections to represent and compare the structure of sugar molecules.
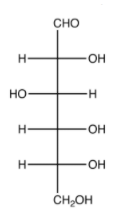
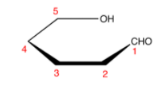
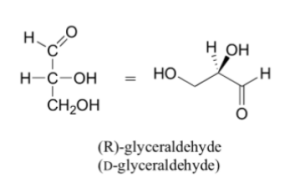
Fischer projections show sugar molecules in an open-chain form.
- In a Fischer projection, the carbon atoms of the sugar molecules are connected vertically and are represented by solid lines.
- In Fischer projection, carbon and oxygen and carbon and hydrogen bonds are shown horizontally.
- The Fischer representation follows this rule,
“vertical bonds point into the plane of the page, while horizontal bonds point out of the page.”
The following diagram shows the Fischer projection for a structure of sugar compound,
Haworth Projection
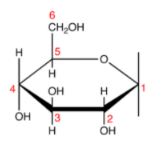
Haworth representation or Haworth projection is a common way of writing and representing chemical compounds. This projection helps in understanding the cyclic structure of various compounds. This method of representing the structure of molecules gives a three-dimensional perspective to the structure and composition of the molecules.
The Haworth representation or Haworth projection of the structure molecules was first used by the British chemist Norman Haworth. The following are the characteristics of Haworth projection for different molecules,
- Carbon is the most common atom generally found in all organic compounds. The carbon atoms are represented with mathematical terms; for example, a carbon atom may be numbered in a Haworth projection. Carbon 1 in the representation is known as the anomeric carbon.
- Hydrogen atoms on carbon are also common; like carbon, hydrogen is widely found in nature. Extra hydrogen atoms are usually not depicted in Haworth’s representation of the molecular structure.
- A thicker line in the representation diagram indicates atoms closer to the observer.
- The group of elements below the plane of the ring in Haworth representation corresponds to the right-hand side of Fischer projection. However, this rule may not always apply depending on the chemical composition of the sugar molecule.
Haworth Representation of Glucose Structure
The following steps should be considered to determine the Haworth formula for the Haworth representation of glucose structure. Consider a D-Glucose compound,
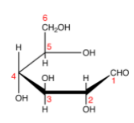
Step1. Draw a Fischer projection for D-glucose (acyclic),
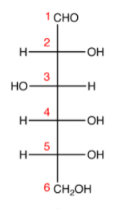
Step 2. Number the carbons,
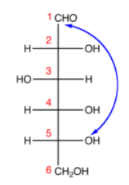
Step 3. Attach the oxygen atom at C-5 from C-1 using a single bond; it will make a pyranose ring structure,
According to the Fischer Projection rule, C-1 is behind the plane of the paper, and C-5 is in front. For the pyranose ring to be planar, both C-1 and C-5 must be behind or in front of the plane.
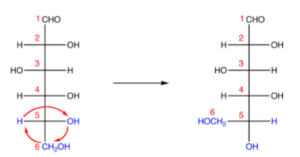
Step 4. Rotate Fischer’s projection in a clockwise direction
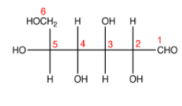
Step 5. Again draw the carbon chain along the horizontal line,
Step 6. Add the compounds from C-2 to C-5,
Step 7. Remove the hydrogen and oxygen atoms from C-1 and C-5,
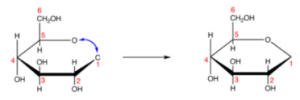
Step 8. Add the remaining bonds at C-1
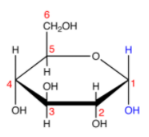
Step 9. Add one hydrogen atom with the bond in C-1,
Step10. Replace the hydrogen atom and hydroxyl group in C-1,
Steps 9 and 10 give the Haworth representation of glucose structure for D-glucose.
Conclusion
Carbohydrates are the most widely found elements in nature; glucose, in particular, is a very common example of carbohydrates; it is primarily found in plants and organic matters. The Fischer and Haward projections or method of representation are the most widely used methods of projecting the molecular structure of chemical compounds. The Haworth representation of glucose structure is important in understanding the alignment and orientation of glucose compounds. The article gives an illustrative description of the Haward representation of glucose structure examples by understanding the structure for D-glucose compounds.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out