Widely known as the king of all acids, sulphuric acid is used in many chemical reactions and industrial processes—making it one of the most commonly used acids globally. Therefore, sulphuric acid preparation is of prime importance due to its high requirement in critical laboratory works and industrial productions such as papers, textile and Rayon, etc. Moreover, sulphuric acid is the backbone of the fertiliser industry. Its preparation is so essential that the amount of sulphuric acid can determine a country’s industrial strength! Let’s see what this highly critical acid is all about in more detail.
What is sulphuric acid (H2SO4)?
Although beneficial, sulphuric acid or the oil of vitriol is a very corrosive and hazardous acid. It is formed by combining three elements of sulphur, water and oxygen with the ratio of 2:1:4; and the chemical formula of sulphuric acid is H2SO4.
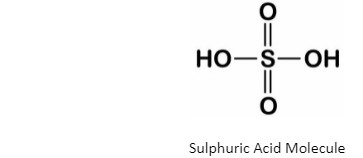
Pure sulphuric acid is impossible to find on earth since it has a very high affinity toward water vapour and gets dissolved in it. Sulphuric acid (H2SO4) structure contains a central sulphur atom surrounded by two oxygen atoms and two hydroxyl groups (OH).
Sulphuric Acid (H2SO4) Molecule
The central sulphur atom forms double bonds with each oxygen atom and has a single bond with the two oxygens in the hydroxyl group. Sulphur forms six bonds and has a formal charge of zero.
The two single oxygen atoms are double-bonded, have two lone pairs of electrons each and a formal charge of zero. Likewise, the hydroxyl groups have a standard charge of zero, and the oxygens in the hydroxyl group have two lone pairs of electrons each.
Sulphuric acid (H2SO4) is a colourless and odourless acid. It is known for its dehydrating properties and is often used as a dehydrating agent. However, it is viscous in nature and miscible with water.
Sulphuric acid is always added to water and not the other way around, as vice versa releases a tremendous amount of heat, causing the mixture to boil and splatter.
Uses of Sulphuric Acid (H2SO4)
Sulphuric acid is the most produced acid worldwide. It has its uses in all industries ranging from fertilisers to the textile industry. The usage of sulphuric acid (H2SO4) is so widespread that it plays a part in almost all manufactured goods we see today. Take a look below:
- Sulphuric acid is used in fertilisers to alter the soil’s pH level or as a dehydrating agent in the mixture.
- It is used to make phosphoric acid.
- It is also used to make fertilisers with calcium dihydrogen phosphate and ammonium phosphates.
- Sulphuric acid is also used to manufacture many chemicals like hydrochloric acid, nitric acid, sulphate salts etc.
- Synthetic detergents are also manufactured using sulphuric acid.
- It also plays a significant role in producing dyes, explosives and medical drugs.
- Petroleum refining also utilises sulphuric acid (H2SO4). It is used to wash away the impurities that may be dissolved in petrol and its other byproducts.
- In the textile industry, rayon cannot be produced without sulphuric acid.
- The batteries that we use almost all the time have sulphuric acid, which facilitates the electricity-producing reaction in the battery.
How is Sulphuric Acid (H2SO4) made?
The best method in present times to produce high concentrations of sulphuric acid is the contact process. The contact process is a four-stage process.
The process is started by forming sulphur dioxide and ends with the formation of sulphuric acid (H2SO4) by dissolving oleum in water:
Sulphur Dioxide formation
Sulphur dioxide is formed at the industry levels in two ways: Either by heating pure sulphur in the presence of oxygen or by roasting the metal sulphides to obtain sulphur dioxide.
Generation of Sulphur trioxide from Sulphur Dioxide
At the second stage, sulphur dioxide is made to react with excess oxygen to yield sulphur trioxide. But the formation of sulphur trioxide is a very sensitive process to temperature and pressure. The reaction is given as:
2SO2 + O2 2SO3
Formation of Oleum
Now that sulphur trioxide is formed, it is dissolved in sulphuric acid to form oleum. The direct addition of sulphur trioxide to water also yields sulphuric acid (H2SO4) but the result is extremely exothermic. Therefore, the direct addition of sulphur trioxide to water yields acid vapours and not liquid sulphuric acid.
On the other hand, oleum can be easily dissolved in water to form highly concentrated sulphuric acid. The reaction is not exothermic compared to directly dissolving sulphur trioxide in water and also allows greater control over the concentration of acid that is formed.
The reaction is given as:
SO3 + H2SO4 → H2S2O7
Dissolving Oleum in Water
Oleum is then dissolved in water to yield sulphuric acid (H2SO4). The reaction is then given as:
H2S2O7 + H2O → 2H2SO4
Conclusion
Sulfuric acid is a clear, greasy liquid with no colour; it dissolves in water with heat discharge. The acid can corrode both metals and tissues. It will scorch wood and most other organic materials on contact, but it is unlikely to start a fire. Inhalation of 15 pounds per gallon density of H2SO4 can have adverse health effects after long-term exposure to low doses or short-term exposure to high amounts. It’s used to manufacture fertilisers and other chemicals in petroleum refining, iron and steel manufacturing, and various other applications.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




