Ethers are organic molecules that have an ether group in their structure. Two alkyl or aryl groups are linked to an oxygen atom to form an ether group. In general, they use the formula R-O-R. Using a bond angle of 104.5 degrees, the C-O distances are around 140 pm. The ether’s oxygen has a greater electronegative charge than the carbon atoms it replaces. As a result, the alpha hydrogens in these chains are more acidic than in normal hydrocarbon chains. An ether has an oxygen connected to two alkyl or aryl groups, as shown here by R and R’. The substitutes don’t have to be the same, but they can be the same.
Structure of ether
Ethers function bent C–O–C linkages. In dimethyl ether, the bond attitude is 111° and C–O distances are 141 pm. The barrier to rotation approximately the C–O bonds is low. The bonding of oxygen in ethers, alcohols, and water is similar. In the language of valence bond theory, the hybridization at oxygen is sp3.
Oxygen is extra electronegative than carbon, for that reason the alpha hydrogens of ethers are extra acidic than the ones of easy hydrocarbons. They are some distance much less acidic than alpha hydrogens of carbonyl groups (including in ketones or aldehydes), however.
Nomenclature of ethers-
Ethers can be given a name in one of two ways. Alkyl groups on either side of the oxygen atom are identified alphabetically, then “ether” is written. Ethyl methyl ether, for example, is an ether in which the oxygen atom is flanked by an ethyl and a methyl group.
The ether is referred to as di[alkyl] ether if the two alkyl groups are the same. Dimethyl ether, for example, is the oxygen-containing ether with an ethyl group on either side of the atom.
Properties of ethers-
- Alkyl groups flank the core oxygen in ethers, making them nonpolar compounds. Oxygen is largely unable to engage in hydrogen bonding because of the massive alkyl groups that surround it.
- Alcohols of the same molecular weight, on the other hand, have higher boiling points.
- Boiling point differences are reduced when alkyl chains become longer in the ethers. There are more Van der Waals interactions, and consequently more electrons, when there are more carbon atoms in the system.
- Ethers are able to establish hydrogen bonds with water because oxygen atoms have two lone pairs of electrons on them. However, ethers are not nearly as polar as esters or alcohols of the same structure.
Reaction of ethers-
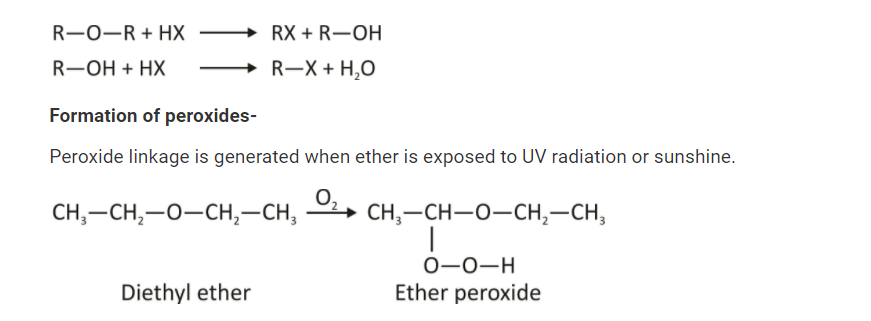
- They are less reactive than alcohol but yet more reactive than alcohols. Even though they don’t readily hydrolyze, acids are able to break them down into an alkyl halide and an alcohol. When exposed to oxygen or air, ethers tend to peroxide. R-O-O-R’ is the basic formula. Either Lewis or bronsted bases, ethers can contribute electrons or take protons in reactions.
- It is possible to synthesise ethers in the laboratory by dehydrating alcohols (2R-OH RO+H2O at high temperature), nucleophilically dissolving chlorides by alkoxides (R-ONa + R’-X RO’+NaX), or electrolytically adding alcohols to alkenes (R2C = CR2 + ROH R2CH(-O)R-R2).
Preparation of ethers-
It is possible to prepare or synthesise Ethers in a variety of different methods. Methods for producing ethers in industry include:
Dehydration of alcohols
Sulfuric and phosphoric acids (protic acids) cause alcohol to dehydrate, resulting in alkenes and ethers under various circumstances. The reaction conditions have an impact on the reaction product’s formation. Sulfuric acid dehydrates ethanol to ethene at 443K, for example. Sulphuric acid at 413K produces ethoxyethane from ethanol, on the other hand.
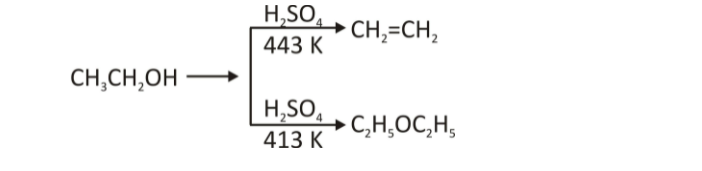
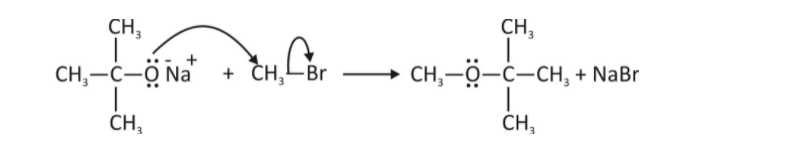
Williamson synthesis
Using this procedure, ethers can be made in the laboratory in both symmetrical and asymmetrical forms. In the Williamson synthesis, sodium alkoxide reacts with an alkyl halide to produce ether. An alkyl halide is attacked by an alkoxide ion via an SN2 reaction. We already know that alkoxides are extremely strong bases, and consequently they play an important role in elimination processes. Williamson synthesis is more productive when dealing with primary alkyl halides.
Example:
Alkyl halide with dry silver oxide-
Ethyl is created by treating alkyl halide with dry silver oxide.
2C2H5Br + Ag2O → C2H5–O–C2H5 + 2AgBr
Ether chemical reactions-
Cleavage of co-bonds in ethers-
Erosion of carbon-oxygen bonds occurs under extreme conditions, such as high acid concentrations and high temperatures, where ether cleaves C-O bonds. There are several examples of chemical reactions that create alcohol and alkyl halides. A second mole of alkyl halide and water are formed as a result of this reaction. Oxygen atoms in ether and alcohol have been shown to be basic, like the oxygen atoms in water. To put it another way, the first step in the chemical interaction between ether and chloride results in protonation. The halide ion’s nucleophilic attack on this protonated ether causes the C-O bond to be broken.
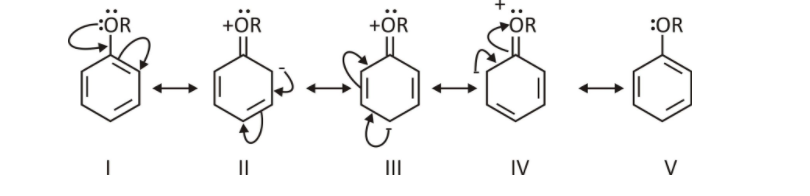
Electrophilic Substitution Reaction-
The presence of alkoxy groups in aromatic ethers activates their aromatic ring toward an electrophilic substitution process similar to phenol (-OR). This alkoxy group has both ortho and para directing properties. Resonance occurs between the lone oxygen pair and the benzene ring, increasing the ring’s electron density in the ortho and para positions, in the case of aryl ethers Electrophile attacks at ortho and para locations are facilitated by this.
Some of the important ethers are:
- Ethylene oxide
- Dimethyl ether
- Diethyl ether
- Dimethoxyethane (DME)
- Dioxane
- Tetrahydrofuran (THF)
- Anisole (methoxybenzene)
- Crown ethers
- Polyethylene glycol (PEG)
- Polypropylene glycol
- Platelet-activating factor
Uses of ethers-
- At low temperatures, dimethyl ether is both a refrigerant and a solvent.
- In surgical anaesthesia, diethyl ether is a typical component.
- As a motor fuel, ether is mixed with petrol.
- It is a common solvent for oils, gums, resins, and other materials.
- The high boiling point of phenyl ether makes it an ideal heat transfer medium.
Conclusion-
About 8-10% of the ethyl ether ingested is thought to be processed in the body, with the rest being eliminated unchanged through the respiratory system. The inducible hepatic microsomal enzyme system, a cytochrome P450-containing monooxygenase system, converts ethyl ether to ethanol and acetaldehyde. To get acetate, which is then used in intermediate metabolism, ethanol and acetaldehyde are quickly oxidised.
Ethers are compounds in which the oxygen atom is sandwiched between two alkyl groups that are either identical or distinct. Ether’s structure, terminology, and properties are all described here. We also discovered the differences between ethers and their alcohol and alkane analogues.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




