Isomerism is the phenomenon in which the molecules have the same chemical formula but different structures. It can be further classified into two categories:
- Structural Isomerism: The molecules with the same chemical formula but different attachments of the atoms with the central metal are called structural isomers, and the phenomenon is called structural isomerism.
- Stereoisomerism: The molecules with the same chemical formula and same attachment with the atoms or same coordination with ligands to metal compared to each other but different spatial arrangements of the atoms or ligands in the space such molecules are called stereoisomers, and the phenomenon is called Stereoisomerism.
Stereoisomers can be further classified into two categories:
- i) Optical isomerism
- ii) Geometrical Isomerism
Optical Isomerism
The compounds have the same chemical formula and same attachment of the ligands with metals but non-superimposable mirror images from each other. It generally occurs in tetrahedral and octahedral complexes. They exhibit optical activity because of the absence of elements of symmetry. Square planar complexes do not show optical isomerism because all square planar complexes have the plane of symmetry until not coordinated with chiral ligands. Optical isomers have physical properties like melting point, boiling point, and density that only differ in stereochemical properties like specific rotation.
- If the coordination compound can rotate plane-polarised light towards the clockwise direction, it’s a laevo isomer denoted as ‘l’ or ‘-‘.
- If the coordination compound can rotate plane-polarised light to an anti-clockwise direction, it’s a dextro isomer demoted as ‘d’ or ‘+’.
Based on the relation of the mirror image of compounds, the optical isomers can be classified as enantiomers and diastereomers.
Enantiomers are the stereoisomers or optical isomers with a non-superimposable mirror image. d and l isomers of a compound are called enantiomers.
Diastereomers are the stereoisomers having non-superimposable, not mirror images. Geometrical isomers are the diastereomers.
Racemic Mixture: It is the equimolar mixture formed by the mixing of two enantiomers (laevo and dextro isomers).
Optical Isomerism in Octahedral complexes:
- Octahedral complexes with the ML6 kind of system don’t show optical isomerism. They are optically inactive (can’t rotate plane-polarised light ) because of the plane of symmetry. ( M is metal and L is ligand )
- MA2B2C2 e.g, [Pt(py)2(NH3)2Cl2]
- M(AA)3 e.g., [Co(en)3]3+
- M(AB)3, where AB is the bidentate ligand, will show optically active isomers. E.g., [Cr(gly)3]
- The Mabcdef type of coordination complexes shows 15 isomers. E.g. [Pt(Cl)(Br)(I)(py)(NO2)(NH3)] will show 15 isomers since 15 isomers also have their mirror images, so a total of 30 compounds will form and all 30 will be optically active.
Optical isomerism in tetrahedral complexes:
- ML4, ML3X, ML2X2 (L and X both are monodentate ligands) types of tetrahedral complexes do not have optical isomerism because they contain a plane of symmetry, so they are optically inactive in nature.
- MABCD will show optical activity because it does not have any plane of symmetry. E.g., Pt(NH3)(py)(Cl)(Br) will show two optical isomerism.
Geometrical Isomerism
Coordination Compounds with the same chemical formula and same kind of attachment of ligands with the central metal but the different geometric arrangements around the central metal.
This kind of isomerism is found in Octahedral and Square planar geometries but not in tetrahedral geometries because no other arrangement is possible in tetrahedral complexes. It occurs in heteroleptic complexes having coordination numbers 4 and 6 because of different possible arrangements around the central metal.
The Geometrical isomers differ in their physical and chemical properties from each other.
- ML4 type of tetrahedral complexes does not show geometrical isomerism since all ligands are in different directions for geometrical isomerism.
- MA3B3 type of octahedral complexes shows facial and meridional isomerism.
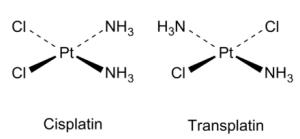
- MA2B2 type of square planer complex shows cis and trans isomers. In the cis isomer, two same types of ligands have 90o angles between them, and in the trans isomer, two same types of ligands have 180o angles between them.
- MABCD type of square planer complex shows three geometrical isomers. 2-cis and 1-trans.
- MA2B4 type of octahedral complex can also show cis-trans isomerism
- Facial and meridional isomers are the coordination complexes of the MA3B3 type. In the Facial isomer, three ligands have cis configuration, while in the meridional isomer, one pair of the ligand is in the trans configuration. E.g., [RhCl3(pyr)3] will show facial and meridional isomerism.
Conclusion
Stereoisomerism is the phenomenon in which the isomers have different spatial arrangements of atoms. Stereoisomerism is categorised as Optical and geometrical isomerism. Optical isomers are optically active because of the symmetry elements, while geometric isomers are not optically active generally because geometrical isomers generally contain a plane of symmetry.
Enantiomers and diastereomers are explained in articles with examples.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out