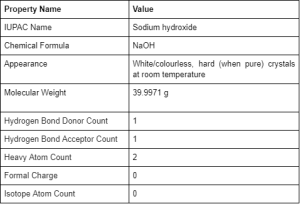
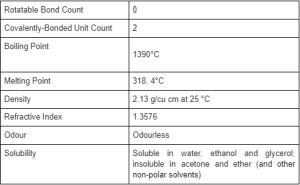
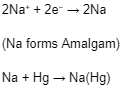Sodium hydroxide is commonly used to produce soaps, paper, dyes, explosives, petroleum products and is used in many other manufacturing processes. Sodium Hydroxide (NaOH) is a white crystalline (at room temperature) ionic compound consisting of sodium cations (Na+) and hydroxide anions (OH–).
Sodium hydroxide is also known as lye and caustic soda. It is a highly caustic base and alkali that has no smell. It is an inorganic compound that is generally used as a solid or diluted in a 50% solution due to its highly corrosive nature.
Physical and Chemical Properties
Some Preparation Techniques
Historically, sodium hydroxide was produced by treating sodium carbonate with calcium hydroxide in a double replacement reaction (causticising).
This technique leveraged the fact that sodium hydroxide is soluble while calcium carbonate is not.
More recent techniques include the following:
- The Castner-Kellner Process
Under this technique, NaOH is prepared by electrolysis of an aqueous solution of Sodium Chloride (also called Brine).
The Castner-Kellner cell is a rectangular tank of steel, with its inside lined with ebonite. Other parts of the cell include:
- A titanium anode
- A cathode in the form of a flowing layer of mercury at the bottom of the tank
- A solution of sodium chloride that serves as an electrolyte
When electric current passes through the brine, +ve and -ve ions migrate towards their respective electrodes, i.e., Na+ ions are discharged at the mercury cathode, and Chlorine is produced at the anode.
The sodium deposited at the mercury cathode forms sodium amalgam, and the chlorine is removed from the top of the cell.
Reaction at Cathode (Negative Electrode):
Na+ ions are discharged in preference to H+ ions due to high voltage.
Reaction at Anode (Positive Electrode):
Formation of NaOH:
The amalgam moves to a different chamber(denuder), where it is treated with water to produce NaOH in a liquid state, which is then converted to solid NaOH by evaporation of the solution.
The NaOH obtained by this method is highly pure, and the process is very efficient.
- Nelson Diaphragm Cell
A porous diaphragm of asbestos or polymer with metal oxides separates the anode (carbon [graphite] or titanium coated with Ru-Ti oxide) and cathode (steel mesh) compartments.
The diaphragm prevents hydroxide ions from entering the anode compartment and chloride ions from entering the cathode compartment. The saturated brine (aqueous NaCl), which is used as the electrolyte, enters the anode compartment, where chlorine gas is then generated.
Reaction at Anode (Oxidation):
Cathode reaction (Reduction):
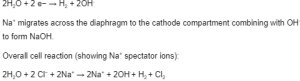
The product contains sodium chloride and sodium hydroxide. NaOH(s) can be crystallised out.
Important Reactions
- Reactions with acids
In an example of neutralisation reactions, sodium hydroxide reacts with protic acids to produce water and the corresponding salts. This type of reaction with a strong acid is highly exothermic. Such acid-base reactions are also used for titrations. However, sodium hydroxide is not used as a primary standard because it is hygroscopic and absorbs carbon dioxide from the air.
- Reaction with acidic oxides
These reactions are often used to scrub harmful acidic gases (like SO2) produced in the burning of coal and other similar industrial processes to prevent their release into the atmosphere. A sample reaction is as follows:
Sodium hydroxide is soluble and is thus used to precipitate transition metal hydroxides that are typically insoluble. Additionally, though not the most preferred choice, NaOH can also be used in the process of saponification.
Uses
- Sodium hydroxide is used to manufacture soaps and a variety of detergents used in homes and commercial applications, including drain cleaners and chlorine bleach.
- Sodium hydroxide is also used to help manufacture medicines and pharmaceutical products like pain relievers, anticoagulants, and cholesterol-reducing medications.
- Sodium hydroxide is used to refine raw materials for wood products such as cabinets and furniture and in wood bleaching and cleaning. It is also a key ingredient in the paper-making and recycling process.
- Municipal water treatment facilities use sodium hydroxide to control water acidity and to help remove heavy metals from water.
Sodium hydroxide is also used in the energy sector has applications in the food processing industry and in multiple manufacturing processes ranging from the the production of rayon, paints, ceramics, glass, dyes to metal cleaning and processing and bleaching.
Health Hazards
Sodium hydroxide toxicity depends on the concentration of the sodium hydroxide solution and the duration of its contact with the tissue.
Concentrated vapours cause serious damage to the eyes and respiratory system.
Contact with the skin can result in dermatitis, loss of hair, and necrosis due to irritation.
Conclusion
Sodium Hydroxide is one of the simplest hydroxides with a multitude of applications across industries. It is a white, odourless ionic compound that is highly corrosive and can also pose potential health hazards with prolonged exposure. Regardless, NaOH is a highly useful ingredient in many industrial processes.
It is also important to know about other hydroxides like KOH (a more common base in saponification), LiOH, Ca (OH)2, etc., and to contrast their behaviours and properties with NaOH.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out