Introduction
In physics and chemistry, the wave nature of electrons is known as an atomic orbital. We can calculate the probability of finding an electron adjacent to the nucleus. The electron spin can be referred to as an indication of angular momentum as well as a quantum property of electrons. Electrons have an angular momentum described by their spin quantum number. In addition to their angular momentum, electrons possess orbital momentum as well.
Orbitals
Orbitals are three-dimensional representations of where electrons are likely to be located around atoms. When the atoms of a molecule form bonds, orbitals combine.
There are multiple orbitals for a quantum atom, which are differentiated by their size, shape, and orientation. In general, the smaller the orbital, the greater the chance of getting an electron close to the nucleus. Various orbitals have different shapes and are designated as s, p, d, and f.
The significance of s and p orbitals is due to their common occurrence in organic and biological chemistry. Atomic orbitals are the wave functions of electrons inside an atom. Furthermore, we can draw boundary surface diagrams with the help of this wave function. Even so, we can clearly describe the electron arrangement in an atom with quantum numbers.
- Number ‘n:’ It is a principal quantum. The ‘n’ orbitals contain a single atom-shell.
- Number ‘l:’ It is an azimuthal quantum. A three-dimensional orbital shape is defined.
- Number ‘ml:’ It is a magnetic orbital quantum. An orbital’s spatial orientation is defined by its coordinate axis.
Let us explore some of the most common types of orbitals and discuss the shape of orbitals.
s orbital:
Around the nucleus of an atom, the s orbital shape has a spherically symmetrical form. Moving away from the nucleus, the energy level increases, which is why the orbitals grow. Near the nucleus, the electron density of a 1s orbital is quite low, but it gradually increases as you move away from the nucleus until it drops below the contour. 2s orbitals are similar to 1s orbitals, but they have an electron density sphere encased within the outer sphere. 3s orbitals have three nodes and are larger. Radial and angular nodes are two types of nodes. A nodal point is a place where it is unlikely for an electron to be found.
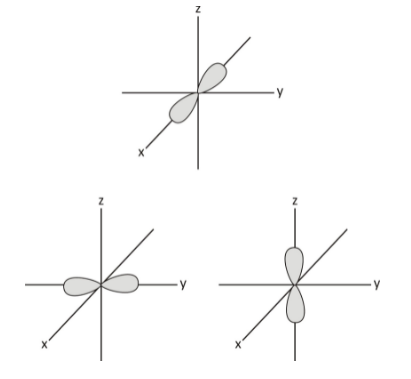
p orbital:
Dumbbell-shaped orbitals make up the p orbital shape. At the centre of the nucleus is the node in the p orbital. As opposed to s orbitals, p orbitals point in a specific direction. There are three orbitals in the p orbital to ensure it can accommodate six electrons at the most. When electrons are at the first energy level, their orbital is the 1s orbital, but as they attain the second energy level, they can also access the 2p and 2s orbitals. Every p orbital has a lobe on each side of the plane running through the nucleus.
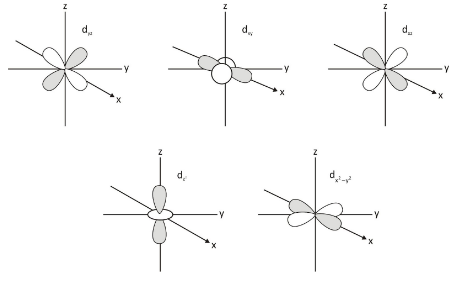
d orbital:
An orbital d can be visualised as a cloverleaf or as plane two dumbbells. dxy, dyz, dxz, dx2–Y2, and dz2 are the names of the five d-orbitals. Although all five orbitals have the same amount of energy, the first four orbitals are quite similar in shape, while the dz2 differs from the others.
Electron Spin
In 1925, Samuel Goudsmit and G.E. Uhlenbeck proposed that the electron has an intrinsic angular momentum referred to as a spin. A classical theory describes the electron as a sphere, but the electron spin theory describes it as a quantum particle. The electron spins either upwards or downwards.
The three steps to identifying an electron spin are:
- A measure of how many electrons are in an atom.
- Illustrating how electrons are arranged in an atom.
- You can use up and down arrows to indicate the direction of electron spin.
Quantum mechanics compute the properties of elementary units such as electrons using spin. Angular momentum, spin quantum number, and freedom degree are all affected by spin direction.
Spin Quantum Number
As a result of their motions and trajectories, electrons can be described by quantum numbers. An atom’s electron position and energy are described by quantum numbers. Quantum numbers can be divided into four types:
- Principal: A principal quantum number is designated by the symbol ‘n.’ It is used to identify an electron’s principal shell. An integer that has a value of one or more can be used as the principal quantum number. Because an atom cannot possess a negative value or no value for its principal shell. ‘n’ can neither be negative nor equal to zero.
- Azimuthal: It is also known as orbital angular momentum. An orbital, defined by its shape, is represented by the azimuthal quantum number, symbolised by ‘l’. Values of azimuthal quantum numbers may indicate s, p, d, or f subshells, which can differ in shape.
- Magnetic: Magnetic quantum numbers determine the number and orientation of orbitals in a subshell, while an azimuthal value determines the magnetic quantum number. It is represented as ml.
- Spin quantum: There is no dependence between the electron spin quantum number and n, l, or ml. The spin direction of an electron is indicated by the value of the number. It is represented as ms. The spin direction of an electron can be determined by using the ms value. Positive values of ms indicate that the electron has an upward spin, also known as a ‘spin up.’ Negative values indicate a downward spin, also known as a ‘spin down.’
These are used to describe all the characteristics of each electron in an atom.
Conclusion
We now understand that with atomic orbitals, we can calculate the likelihood of finding electrons around a nucleus. There are four different kinds of orbitals (s, p, d, and f), each with a different size and different maximum capacity of holding two electrons. Furthermore, as the electron spins around its axis, it is considered to be electron spinning. There is a fourth quantum number, called the spin quantum number, which describes how an electron spin is oriented in space.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





