In the study of organic chemistry and organic compounds, amines play a very crucial role. Amines are derivatives of ammonia, in which one or more than one hydrogen is simply substituted with an alkyl group or aryl group, and the classification of amines into primary, secondary and tertiary is done on the basis of the fact that number of hydrogen atom have been substituted by the alkyl or aryl group. In simple terms, it can be defined as the amines containing two alkyl groups and one other group, say “X”. Here, “X” can be anything but an alkyl or aryl group. Secondary amines possess most of the properties as intermediate, which means neither greatest nor least. For instance, the intermolecular bond strength is less than primary amine and more than tertiary amine; more specifically, it is the compound that is formally derived from ammonia after replacing two hydrogen atoms with hydrocarbyl groups.
Examples of Secondary amines are
Dimethyl amine (CH3)2NH, Diphenylamine (C6H5)2NH.
Nomenclature of secondary amine :
In order to have a uniformity of names of compounds, the International Union of Pure and Applied Chemistry, IUPAC, came up with a universal pattern for naming organic compounds. Thus, all the organic compounds are named as per IUPAC nomenclature norms, and the amines are also named similarly, and the letter “e” is replaced by the word “amine” as IUPAC nomenclature rules do not allow two vowels together.
The number of alkyl groups attached to the Nitrogen in a secondary amine is two. The root name of the secondary amine is based on the longest chain, which is assigned to the Nitrogen. For IUPAC nomenclature, the chain is numbered in such a way that the amine unit is given priority and gets the lowest possible number. The second alkyl group will be considered as a substituent.
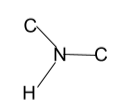
Structure of Secondary Amine
Physical properties of secondary amines:
Boiling point: The boiling point of a compound depends on several prominent factors: hydrogen bonding and bond strength. For secondary amines, there is hydrogen bonding and bond strength more than tertiary amines but less than primary amines, so the boiling of secondary amines is more than tertiary amines but less than primary amines.
Solubility: It depends on the hydrophobic part, polarity and hydrogen bond of the given amine with water. The hydrophobic part is more than primary but less than tertiary, so the solubility of secondary amine is also in between, which is less than primary amine and more than secondary. However, practically most of the higher secondary amines are insoluble in water and soluble in organic solvents.
State: Except for primary amines, both tertiary and secondary amines are in the gaseous phase due to weaker bonding as they have lesser hydrogens, and thus hydrogen bonding is not much effective in secondary amine and doesn’t exist in tertiary amine.
Chemical properties of secondary amines :
Basicity: Amines are basic in nature in general; tertiary amine is most basic, followed by secondary and primary. This trend is observed because basicity is directly proportional to +I effect, and electron-donating groups show this effect. The +I effect increases the overall electron density of the compound to which it is attached. There are more electron-donating groups in the tertiary amine, and in the primary amine, there are fewer electron-donating groups than the secondary amine. Hence, the order of basicity is lowest in primary amine and highest in tertiary amine.
Synthesis of secondary amine:
A secondary amine is synthesised using a coupling reaction; in this reaction, a catalytic system is generated in-situ from a tetranuclear complex with a catechol ligand and enables a direct deamination coupling of two primary to form secondary amines. There is an analogous coupling of aniline with primary amines.
The test to distinguish between primary, secondary and tertiary amines is known as the hinsberg test; this test is based on the formation of sulfonamides. In this test, benzene sulfonyl chloride is reacted with an amine. If the product is formed, it is either a primary amine or secondary amine but not a tertiary amine. Thus it rules out the possibility of a tertiary amine. In order to distinguish between primary and secondary amines, it is checked whether the product dissolves in aqueous sodium hydroxide. If it dissolves, it is a primary amine, and if not, then secondary amine.
Uses of secondary amine:
The secondary amines are extensively used as one of the critical reagents for carbon-nitrogen bond formation and are thus used in organic synthesis and medicinal chemistry.
Conclusion :
Secondary amines are the amines having two alkyl groups, and they have most of the properties as an intermediate. The secondary amines are very useful and are widely used as an essential reagent in organic synthesis. A coupling reaction synthesises it.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out