Thermodynamics is the study of relationships between heat and energy such as potential, kinetic, light, mechanical, etc.
Generally, we study the following laws of Thermodynamics:
- Zeroth law of thermodynamics
- The first law of thermodynamics
- The second law of thermodynamics
- Third law of thermodynamics
Here we understand the second law of thermodynamics in detail with examples.
The second law states
“Spontaneous natural processes increase entropy overall.”
We can understand this as that,
“Heat can spontaneously be conducted only from a higher-temperature region to a lower-temperature region.”
An example of irreversibility is in the transfer of heat by conduction. It is a known fact that when two bodies of different temperatures, then heat always flows from the hotter body to the colder body.
Entropy statement of Second law of thermodynamics
“In all the spontaneous processes, the entropy of the universe increases.”
Kelvin Planck’s statement
“It is impossible to construct a device (operating in a cycle) that can work on a single heat source and convert all of its heat completely into work.”
Clausius’s statement
“Heat cannot itself flow from a colder body to a hotter body.”
Explanation:
The second law states,
There exists a useful state variable known as entropy S.
The change in entropy of a closed system( ΔS )is equal to the heat transfer ( ΔQ )divided by the temperature T.
Δ S = Δ Q / T
For a given physical process if the process can be reversed, the total entropy of the universe i.e., the system and the surrounding, remains constant.
If we denote the initial and final states of the system by “i” and “f” respectively then:
Sf = Si (reversible process)
The second law states that if the physical process is irreversible, the combined entropy of the system and the environment should be increased.
For an irreversible process, the final entropy should be higher than the initial entropy.
Sf > Si (irreversible process)
Example :
A hot thing is put in contact with a cold thing, they both achieve the same equilibrium temperature. After separating the items, the equilibrium temperature remains and does not naturally return to its initial temperatures. This is an irreversible process.
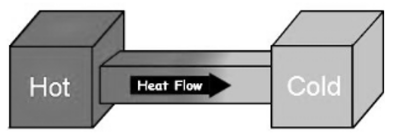
The Second law of thermodynamics in terms of entropy
“The complete entropy of the system and its surroundings increases in a spontaneous process.”
It can be thermodynamically represented as
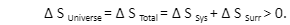
Gibbs free energy and spontaneity of the process:
OR
The criterion of spontaneity in terms of Gibbs free energy is the same as that laid down by the second law of thermodynamics:
The total entropy changes for a system and its surrounding with a process can be written as:
∆ S Total = ∆ S Sys + ∆ S Surr .
By Second law, for spontaneous process,
∆ S Total > 0.
If +∆H is the enthalpy increase for the process or a reaction at constant temperature (T) and pressure, the enthalpy decrease for the surroundings will be -∆H.
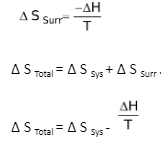
T ∆ S Total = T ∆ S Sys – ∆ H.
-T ∆ S Total = -T ∆ S Sys + ∆ H.
-T ∆ S Total = ∆ H -T ∆ S Sys
By Gibbs equation
∆G= ∆H – T∆S
Now by the above two equations
∆G= -T ∆ S Total
As ∆ S Total increases ∆G decreases.
For spontaneous process, ∆ S Total > 0
This is according to the second law of thermodynamics.
∆G < 0.
So, in a spontaneous process, Gibbs free energy decreases (∆G < 0), while entropy increases (∆ S > 0).
Therefore for a non-spontaneous process, Gibbs’s free energy increases (∆G > 0).
It can be interpreted that for a process at equilibrium, ∆G = 0,
Conclusion
- For the spontaneous process ∆G < 0.
- For the non-spontaneous process ∆G > 0.
- For the process at equilibrium ∆G = 0.
Examples of the Second Law of Thermodynamics:
- Water always flows from a higher level to a lower level spontaneously.
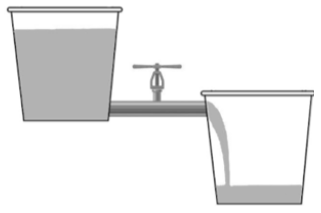
In the above image, we can see that water always flows downward and it never goes upward automatically.
In the above image, we can see that water always flows downward and it never goes upward automatically.
- A hot tea becomes cold spontaneously but it cannot become hot again automatically.

- Air leaks from the balloon by itself is an example of a spontaneous process:
We know that air never goes inside the balloon by itself. This can be explained based on the Entropy statement of the second law of thermodynamics.
4. Two gases will mix automatically by themselves is an example of a spontaneous process:

From the above diagram, it is clear that the gases will get mixed by themselves, which is an example of the second law of thermodynamics.
- Stone falls on the ground by itself is an example of the spontaneous process:

The above process of falling of stone indicates that it is a spontaneous process and the entropy of the universe increases.
- Ice melts by itself is an example of a spontaneous process:

The above process of melting ice indicates that it is a spontaneous process and the entropy of the universe increases.
- A gas occupies the entire volume of the container:
If we insert gas in the closed container, the gas will occupy the entire space of the container.
8. After burning an incense stick in a room, the fragrance spreads in the entire room. It is a spontaneous process and the entropy of the universe increases.

Example based on Kelvin Planck’s statement
- Cars and bikes engine is an example of the second law of thermodynamics:

As we know that Kelvin Planck’s statement:
“It is impossible to create a device (present in a cycle) that can work on a single heat source and converts all of its heat completely into work.”
In a car and bike engine, there is a higher temperature reservoir (where the heat is produced) and a lower temperature reservoir (where the heat is released).
So according to Kelvin Planck’s statement, there should be at least two thermal reservoirs (one at higher temperature and the other at lower temperature) for the engine will work.
Example based on Clausius’s statement
According to Clausius’s statement:
“Heat cannot itself flow from a colder object to a hotter object.”
- Refrigerator using electricity to change the direction of heat flow
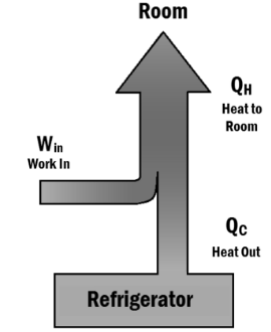
In the refrigerator, the heat is travelling from the lower temperature (inside the refrigerator) to the higher temperature (outside the refrigerator). The heat flow is possible because of a supply of external electrical energy to this refrigerator. This electrical energy is used in the compressor of the refrigerator to produce mechanical work.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





