Chemical bonding is the attraction between atoms that results in a chemical bond. The bond between two atoms is what causes a molecule to be a substance, and without a chemical bond, a molecule would not be a substance.
A chemical bond is a strong interaction that exists between two or more atoms. The bond holds the atoms together and gives them a strong enough attraction to each other to allow them to be separated. This separation is known as diffusion, and the atoms can then be moved around, or changed into different chemical compounds.
Resonance in Chemical Bonding
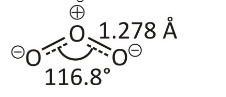
A single Lewis formula cannot describe delocalised electrons within molecules. Polyatomic ions that a single Lewis formula cannot clarify are called resonance. Several resonance structures depict a molecule or ion with such delocalised electrons. When resonance structures differ, it’s crucial to determine which one(s) best describes the actual bonding. The formal charge can predict which resonance structures are most likely to be preferred. The situation is with ozone (O₃), an oxygen allotrope with a V-shaped design and a 117.5° O–O–O angle.
Nuclear magnetic resonance is based on many nuclei having spun, and all nuclei are electrically charged. When an external magnetic field is applied, energy can be transferred to a higher energy level from the base energy.
What are Resonance Structures?
The Lewis structure sets that describe the electron’s delocalisation in a molecule or a polyatomic ion are known as resonance structures.
Chemical bonding can be described using resonance structures in such circumstances.
Characteristics of Resonance
- It doesn’t exist in the actual world.
- The bond length in identical resonating structures is the same.
- The resonance hybrid has the least amount of energy hence most stability.
- The more resonance and resonance energy there is, the more stable the structure becomes.
- Resonance is a theoretical idea that has yet to be proven experimentally.
Rules for Delocalization and Resonance Structures
- Resonance structures should contain the same number of electrons; no electrons should be added or subtracted. (Count the number of electrons to see how many there are.)
- Each resonance structure follows the Lewis Structures writing rules.
- The structure’s hybridisation must remain constant.
- The structure’s skeleton cannot be altered (only the electrons move).
- The number of lone pairs in a resonance structure must be the same.
Identifying Viable Resonance Structures Using Formal Charges
While every resonance structure adds to the molecule’s entire electronic structure, their contributions may not be equal. One way of determining the viability of a resonance structure and its relative significance among other structures is to assign formal charges to atoms in molecules.
Use the following formula to find the standard charge on a particular atom in a covalent species:
Formal Charge = (number of valence electrons in the free orbital)−(number of lone-pair electrons) − ½ (number bond pair electrons)
Rules for Estimating Resonant Structure Stability
- The more covalent bonds there are, the more stable the system is since more atoms will have complete octets.
- The structure with the fewest formal charges is more stable.
- The structure with the least formal charge separation is the most stable.
- Positive charges on the most electropositive (least electronegative) atom are more durable.
- Equivalent resonance forms have the same level of stability and contribute equally (e.g., benzene)
Bond Nature and Stability (Resonance)
How does the nature of a bond affect the stability of a molecule?
The answer is resonance.
Resonance involves the breakage of old bonds and the formation of new bonds between atoms. The structures formed due to resonance always exist simultaneously in every specimen of the compound and contribute to the properties exhibited.
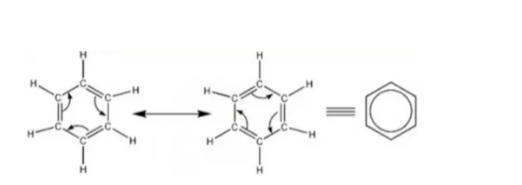
The nature of the bond helps us understand resonance. This is because the ionic bonds and covalent bonds break up differently. A covalent bond breaks up by giving each atom an electron. An ionic bond breaks by forming a cation and an anion.
A covalent bond is also much more stable than an ionic bond, and hence, more difficult to break. Thus, understanding the nature of the bonds in a compound helps us understand the stability it may have.
Conclusion
A single Lewis formula cannot describe delocalised electrons within molecules. Polyatomic ions that a single Lewis formula cannot clarify are called resonance. Several resonance structures depict a molecule or ion with such delocalised electrons. When resonance structures differ, it’s crucial to determine which one(s) best describes the actual bonding. The formal charge can predict which resonance structures are most likely to be preferred. We have studied the resonance structure of several molecules and polyatomic ions in detail.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





