The Reimer-Tiemann reaction is a type of substitution reaction named after two chemists Karl Reimer and Ferdinand Tiemann. The reaction is employed for the ortho-formylation of C6H5OH (phenols).
Reimer-Tiemann reaction is a chemical reaction used for producing phenolic aldehydes under the action of chloroform and caustic alkaline on phenol in industries. Reimer-Tiemann reaction is an aromatic substitution reaction with great industrial importance.
The Reimer-Tiemann response uses a dichlorocarbene precursor patch similar as chloroform, an alkali hydroxide, and a protic detergent to convert a phenol to an ortho-formyl phenol. The first step removes the hydrogen from chloroform, generating a trichlorocarban ion which loses a Cl+ to shape dichlorocarbene. Phenol is too deprotonated by the base which is used as a reagents such as NaOH or KOH , which helps in delocalizing the electrons into the ortho position of the ring, which attack the carbene. The internal proton is transferred, and release of a chloride ion forms a C-C double bond. Hydroxide adds to it, performing in the release of the last chloride ion. Tautomerization produces the final product.
Definition:
When phenol is processed with chloroform inside the ubiquity of NaOH, a CHO group is added at ortho function of the benzene ring. This reaction is understood as the Reimer-Tiemann reaction.
The intermediate form which is substituted benzyl chloride is hydrolysed within the presence of alkali to supply salicylaldehyde as a product.
Reimer-Tiemann reaction also can be termed because the chemical reaction is used for the ortho-formylation of phenols.
The bond formation of the product of Reimer-Tiemann Reaction is :
Ph-CH=O.
Chemical Reaction:
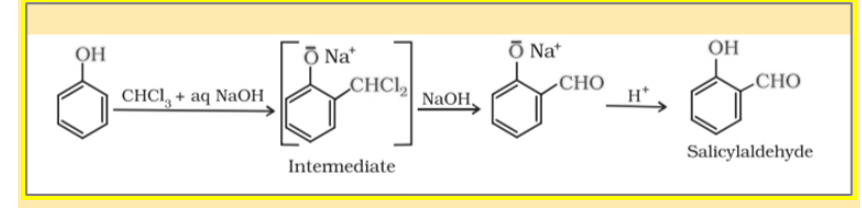
In this reaction, a mixture of both ortho and para isomers is obtained in which the ortho isomer is the major product. If any one of the ortho positions of the benzene ring is occupied, the para-isomer is the major product.The two isomers are separated by fractional distillation, in which Unreacted phenol and the ortho-isomer distill over leaving in the back of the para-isomer. Ortho products are major due to two reasons-
1.Probability factor
- H-bonding in the final salicylaldehyde
Conditions for Reimer-Tiemann reaction:
- Must be carried out in a Biphasic solvent system. A Biphasic mixture is an aggregate of immiscible stages which commonly includes a natural solvent and an aqueous phase.
- The two reagents are brought together by rapid-fire mixing, phase transfer catalysts or the use of 1,4-Dioxane which is an emulsifying agent.
- This reaction is effective when other hydroxy – aromatic compounds are used such as naphthols.
- Heat is necessary for initiating the process of the reaction.
- Once the reaction has begun, it is highly exothermic.
Mechanism of Reimer-Tiemann:
- Deprotonation of the chloroform is carried by the explosively introductory aqueous hydroxide solution, which gives the chloroform carbanion.
- This chloroform carbanion readily undergoes nascent (alpha )elimination, and gives dichlorocarbene as the product.
- The phenol reactant is then deprotonated by the NaOH(aq), giving a negatively charged phenoxide.
- The negative charge formed is now delocalized into the benzene ring, resulting in it being far more nucleophilic.
- Delocalized benzene ring results in a nucleophilic attack on the dichlorocarbene, forming an intermediate dichloromethyl substituted phenol named carbene.
- The intermediate so ordered is subordinated to introductory hydrolysis to eventually achieve the conformation of the asked ortho-hydroxybenzaldehyde.
The mechanism of the chemical equation is as follows:
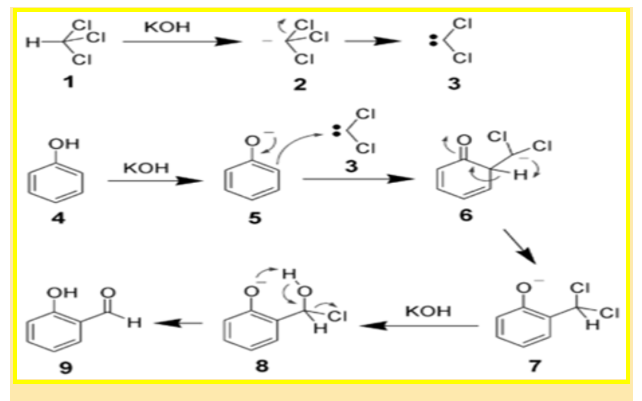
Therefore, the given phenol as a reactant is transformed into an ortho-hydroxy benzaldehyde as a product of the use of chloroform, a base and acid .
Notably, the carbene is largely electron-deficient due to the electron-withdrawing nature of its two chlorine groups. That’s the reason it is explosively attracted to the phenoxide which is rich in electrons. The interaction or the commerce favors ortho-formylation of a selective and picky nature.
Application of Reimer-Tiemann
« Reimer-Tiemann reactions are often slightly altered to yield phenolic acids like 2-hydroxybenzoic acid, by substituting the chloroform with carbon tetrachloride.
« Used mainly for the ortho formulation of the phenols.
« Through Reimer-Tiemann reaction the direct formylation of the aromatic compounds is often done safely and is the easiest method for the formylation , because it is the only method that doesn’t require acidic or anhydrous conditions.
Scope of Reimer-Tiemann
The Reimer–Tiemann reaction is effective for other aromatic hydroxy compounds, like naphthols. Heterocycles which are rich in electrons like pyrroles and indoles are also found to react. Dichlorocarbene can undergo this reaction with alkenes and amines to respectively form dichlorocyclopropanes and isocyanides. As such, this reaction may be infelicitous for substrates bearing these functional groups. In addition, numerous composites can’t repel being heated in the presence of-OH group.
Variations
The Reimer–Tiemann reaction are often altered to yield phenolic acids by substituting the chloroform with carbon tetrachloride as an example , the altered reaction with phenol would yield 2-hydroxybenzoic acid instead of the expected product, salicylaldehyde.
Comparison to other methods
The direct formylation of aromatic compounds are mostly accomplished by the various different methods like the Gattermann reaction, Gattermann–Koch reaction, Vilsmeier–Haack reaction, or Duff reaction and many more.However, in terms of ease and safety of operation, the Reimer–Tiemann reaction is usually the foremost advantageous and profitable route chosen in chemical synthesis. Out of the reactions referred to before, the Reimer–Tiemann reaction is that the most effective path now no
longer, requiring acidic and/or anhydrous conditions.Additionally the Gattermann-Koch reaction is not relevant to phenol substrates.
Things to remember:
- Organic chemical reaction mainly used for the preparation of salicylaldehyde from phenol.
- It is a substitution reaction.
- In the Reimer and Tiemann reactions, the reactants are both phenol, CHCl3 and alkali, and phenol, CCl4, and alkali. When phenol, Chloroform, and alkali are used, the product obtained is salicylaldehyde. When phenol, CCl4, and alkali are used, the product obtained is salicylic acid.
Note:
(i) In these reactions, the phenoxide ion formed will show a mesomeric and inductive effect so the reaction might take place at ortho / para position. But as we know, the +I effect decreases with increasing distance, therefore the ortho position will be electron rich and the incoming electrophile will attack the ortho position. So, formulation will take place at the ortho position.
(ii) The simple alkyl groups, methyl groups and primary alkyl groups always react by the SN2 mechanism. This is due to the fact that if it proceeds via the SN1 pathway, the cations formed will not be stable.
Conclusion
The response of Reamer-Tiemann is an electrophilic substitution reaction which involves the treatment of phenol with chloroform in NaOH(aq) solution result followed by the acid hydrolysis, which leads to the conformation of salicylaldehyde (ortho-hydroxybenzaldehyde) as a chief product of the response.
When a derivative of phenol reacts with sodium hydroxide, it forms a phenoxide ion which is much more stable and rich in electrons than phenol in the presence of chloroform to produce salicylaldehyde.
We have also seen the mechanism of Reimer –Tiemann in which the intermediate carbene is hydrolysed to get salicylaldehyde.
Related Pages
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





