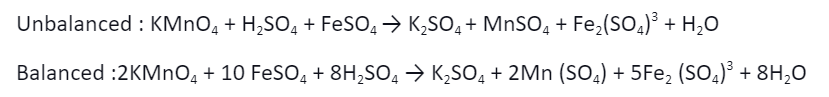All reactive species can be divided into two types of reactions: reductions and oxidation. In a redox reaction or oxidation-reduction reaction, oxidation and reduction always happen simultaneously. The oxidising agent is the material being reduced in a chemical process, while the reductant is the substance that is being oxidised.
A redox reaction is a reaction in which electrons are transferred between two reactants that are involved. Changes inside the oxidation states of a reacting species can be used to identify this transfer of electrons. Oxidation is defined as a loss of electrons and the consequent rise in the oxidised form of a given reagent. Reduction is the process of gaining electrons and decreasing the oxidation state of a reactant.
Oxidants are particle entities that tend to suffer a reduction in redox processes. A reducing agent is an electron-donating species that tend to hand over electrons. Oxidation is a common occurrence in several species. Any redox process can be decomposed into two half-reactions: oxidised half-reaction and the reductive half-reaction.
Redox reaction types
The following are examples of redox reactions:
Reaction of decomposition
Reaction of combination
Reaction to displacement
Reactions of disproportion
Reaction of decomposition
The reaction of decomposition entails breakdown of a molecule into other compounds. Some examples of these types of reactions are as follows:
2NaH 2Na + H2
All of the reactions above lead to the breakdown of minor chemical compounds in-
AB→ A + B.
However, one exception proves that not all decomposition reactions are redox reactions:
CaCO3 → CaO + CO2 is one such example.
Reaction of combination
These reactions are inverse of decomposition processes in that they combine two chemicals to generate a single compound with the formula A + B→ AB.
Consider the following scenario:
4 Fe+ 3O2 →2Fe2O3
Reaction of displacement
An atom or an ion of a compound is replaced by an atom or an ion of another element in this reaction. It can be represented as X + YZ → XZ + Y. Displacement reactions can also be divided into two types:
Displacement of non-metals
Displacement of metals
Another metal displaces a metal present in the compound in this reaction. Several reactions are used when pure metals are extracted from their ores in metallurgical procedures.
For example, CuSO4+Zn→Cu+ZnSO4.
Displacement of non-metallic materials
We can detect a hydrogen displacement reaction and rare oxygen displacement events in this type of reaction.
Reactions of disproportion
Disproportion reactions are those that involve only one reactant being oxidised and reduced.
P4 + 3NaOH + 3H2O → 3NaH2PO2 + PH3 is an example.
Redox reaction examples
This section contains a few redox reactions and their oxidation/reduction half-reactions.
Hydrogen and Fluorine Reaction (Example 1)
In the reaction between hydrogen and fluorine, the hydrogen is oxidised while the fluorine is reduced. The following is an example of a response.
H2 + F2→2HF
H2 →2H+ + 2e– is the oxidation half-reaction.
F2 + 2e– →2F– is the reduction half-reaction.
After that, the hydrogen and fluorine ions unite to form hydrogen fluoride.
Zinc and Copper reaction (Example 2)
This is a metal displacement reaction in which zinc displaces the Cu2+ion in a copper sulphate oxidising solution to produce copper metal, as seen in the response below:
Zn (s) + CuSO4 (aq) → ZnSO4 (aq) + Cu (s)
The half-reaction of oxidation can be represented as Zn → Zn2+ + 2e–
2Fe2+ + H2O2 + 2H+ → 2Fe3+ + 2H2O
In a redox process, copper is replaced from the copper sulphate by zinc.
Iron and Hydrogen Peroxide reaction (Example 3)
When an acid is present, hydrogen peroxide oxidises Fe2+ to Fe3+. The following response occurs:
2Fe2+ + H2O2 + 2H+ → 2Fe3+ + 2H2O
Fe2+ →Fe3+ + e– oxidation half-reaction
H2O2 + 2e– →2 OH–
As a result, the hydroxide ion generated by hydrogen peroxide reduction joins with the proton provided by the acidic medium to form water.
Reactions of oxidation and reduction
Oxidation reaction
Adding oxygen or a more electron-deficient component to a compound or eliminating hydrogen or a more electrophilic element from a material is called an oxidation reaction.
Some instances of oxidation processes are as follows:
2S(s) + O2 (g) → SO2 (g) CH4 (g) + 2O2 (g) → CO2 (g) + 2H2O (l)
Oxidation process
Adding oxygen or the more electron-deficient component to a compound or eliminating hydrogen or a more electrophilic part from a material is called an oxidation reaction.
Reducing agents of significant importance include:
● All metals, such as Na, Zn, Fe, and Al
● Quasi-metals, such as C, H, S, and P Hydracids, including HCl, HBr, HI, and H2S
● There are not many compounds with a component in the lower oxidised form. FeCl2, FeSo4, SnCl2, and Hg2Cl2 are some examples
● NaH, LiH, CaH2, and other metallic hydrides
● HCOOH, an organic chemical
In the presence of water, lithium is the most potent reducing agent, but in the absence of water, caesium is the most powerful reducing agent. H2O2, SO2, HNO2, NaNO2 are oxidising as well as reducing agents.
Reduction reaction
Reduction reactions, like oxidation events, are defined as electron gains. During a chemical process, any material that acquires an electron is reduced.
The reducing reaction is defined as adding hydrogen or a more electropositive component to material or removing more electronegative or oxygen components.
Here are some oxidation and reduction example to consider:
CH3CH3 → 2CH2CH2 (g) + H2 (g) (g)
2FeCl2 (aq) + 2HCl → 2FeCl3 (aq) + H2 (g) (aq)
Looking closely at the above oxidation and reduction examples, it can be seen that they all have both reduction and oxidation reactions.
As the electron-deficient component chlorine is eliminated from FeCl3, it undergoes a reduction process. Hydrogen is oxidised due to chlorine, an electron-deficient element in the same process.
Half-reaction reduction potential
A reference electrode potential exists for each half-reaction, making it a redox reaction. This potential is the voltage generated by an electrolytic system in which the base reaction is regarded as the half-reaction, and the anode is a conventional hydrogen electrode.
Their reducing potentials are the voltages produced by the half-reactions (denoted by E0 red). For oxidising agents that are stronger than H+, the reduction potential of a half-reaction is positive, and for those that are weaker, it is negative.
F2 has a reducing power of +2.866 V, while Zn2+ has a reducing power of -0.763 V.
If a component is in its highest possible oxidised form in a mixture. It can act as an oxidiser. KMnO4, K2Cr2O7, HNO3, H2SO4, HClO4 are some examples.
When an element in a compound is in its lowest oxidation state, it can act as a reducing agent. H2S, H2C2O4, FeSO4, SnCl2 are some examples.
If a highly electronegative element is in its highest oxidation state, the chemical will operate as an oxidising agent.
When an electronegative component is in its most minor oxidation state, it functions as a reducing agent.
Conclusion
Redox processes are commonly used to store and release physiological energy. The reduction of carbon dioxide into sugar and the oxidation of water into oxygen molecules are both parts of photosynthesis. Sugars are oxidised in respiration’s reverse reaction to form carbon dioxide and water. Redox reactions are also employed in the electroplating process, which involves depositing a thin substance layer to an item. It is used in the manufacturing of gold-plated jewellery. It is engaged in the separation of metal in the ore. The smelting of metal sulphides in the oxidising and reducing chemicals is one such instance. Learning about redox reactions, types of redox reactions, decomposition reactions, combination reactions, displacement reactions, disproportionation reactions and balancing redox reactions can help in understanding some of these applications.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out