Introduction
Phenol is mono-hydroxy-benzene, an organic compound; it is the specific name for carbolic acid, with the molecular formula C6H5OH. It is solid white, and it needs to be handled with care as it can cause chemical burns. It is a weak acid, easily soluble in water.
Phenol is a chemical compound that contains an -OH group attached directly to an aromatic ring system. It is widely used to synthesise plastic and related materials. It is a substance with numerous health benefits and a solution for many problems in our daily lives. Products containing phenol are used as chemical peels that help treat severe pain.
What are the sources of phenol?
The conversion of coal leads to gaseous or liquid fuels. Earlier phenol was mainly manufactured from coal tar; recently, it is manufactured in bulk from petroleum. It is easily found in oil refinery wastes. It is also obtained from essential oils of plants. Phenols based in plant-based compounds are called anti-oxidants. Phenol can also be obtained from benzene through hydrolysis of chlorobenzene.
Phenolic compounds are also found in water through the decomposition of dead plants and animals as an organic aquatic waste. The highest phenol concentration is found in coastal areas near human population areas. It can also be created through the process of oxidation and synthesis. Phenolic compounds are also found naturally in fruits and wines. Red grapes contain high amounts of phenol. Polyphenols are compounds that we get through plant-based food such as cloves, berries, beans, and nuts that are raw and roasted.
Nomenclature of Phenol
According to IUPAC (International Union of Pure and Applied Chemistry), the parent name of phenol is benzene. The hydroxyl group attached to the benzene ring is positioned and numbered. If more than one hydroxyl is present, then di, tri and tetra such numerical prefixes represent the number of a hydroxyl group attached.
Substituted phenol compounds are conveyed using the prefix ortho (o-) (1,2 – distributed), meta(m-) (1,3– distributed), or para (p-) (1,4 – distributed), based on the placement of the substituent from the hydroxyl group. There are different rules for naming a compound whenever other molecules are combined with phenol.
As different molecules are attached to the compound, numbering starts with the OH group of the phenol. Then numbering is done according to the highest preferences of different molecules. The numbering is done either in the clockwise or anticlockwise direction.
How is phenol synthesised? Benzene to phenol mechanism!
Today, most of the phenol used is synthesised using the benzene to phenol method. The most two standard processes are either through hydrolysis of chlorobenzene, also known as the Dow process, or oxidation of isopropyl benzene (cumene), also known as the Hock process.
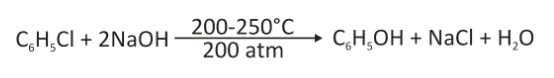
The Dow process
In this, benzene is easily converted to chlorobenzene. Chlorobenzene is hydrolyzed by a strong base at high temperatures to get phenoxide salt, which is acidified to phenol. In this process, chlorobenzene is reacted with dilute sodium hydroxide at a temperature of about 300 degrees celsius and 3000 psi pressure. Chlorobenzene is hydrolysed to phenol using alkali or steam.
The Hock process
With the help of propylene and an acidic catalyst, benzene is converted to isopropylbenzene (cumene). Phenol and acetone are obtained through acid-catalysed rearrangement of hydroperoxide (cumene hydroperoxide) by oxidation.
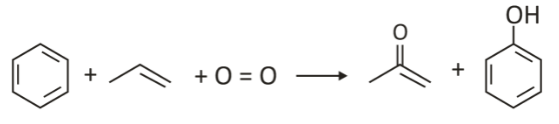
The Hock process uses relatively mild conditions and low-cost raw materials. Although this process looks more complicated than the Dow process, it is preferable as it produces two valuable high-demand products: acetone and phenol.
An ideal temperature and appropriate catalysts should be used while conducting these reactions. Phenol can be produced through various methods, but the most commercial method is one with the help of cumene.
Conclusion
Phenol is a chemical compound that contains an -OH group attached directly to an aromatic ring system. It is widely used to synthesise plastic and related materials.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





