The presence of this organic molecule can be observed in a variety of vegetables and plants. A dicarboxylic acid with the condensed formula HOOC-COOH, it is the simplest of the dicarboxylic acids and has an acidic strength greater than acetic acid. Excessive ingestion of oxalic acid might be hazardous to your health. It is created as a result of the oxidation of carbohydrates in the body. A synthetic version of this compound can also be made in the laboratory by oxidising sucrose in the presence of nitric acid in the presence of a catalyst such as vanadium pentoxide.
Oxalic acid is composed of two polymorphic structures, the first of which appears as a white crystalline solid that, when dissolved in water, transforms into a colourless solution. It is a reducing agent that can also be employed as a chelating agent when combined with oxalate, which serves as its conjugate base.
Formula for Oxalic Acid
Oxalic acid is a dicarboxylic acid with the molecular formula C2H2O4 that is used in food processing. Oxalic acid is found in the cell sap of the plant species Oxalis and Rumex in the form of potassium and calcium salts, respectively.
Oxalic acid is a weak acid that will only partially ionise when present in aqueous solution. Oxalic acid has two protons that are acidic. The initial ionisation results in the formation of HC2O4–, a weak acid that will eventually ionise.
Oxalic acid is one of the most potent organic acids available, capable of dissolving carbonic acid and a wide range of other acids from their salts. Oxalic acid is formed by the action of either a hydrate of potash or a nitric acid on the majority of organic molecules found in the natural environment. It is referred to as diprotic acid in some circles.
Oxalic Acid Structure
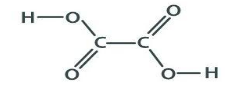
It should be noted that oxalic acid exists in two distinct polymorphs when it is in its anhydrous state. When oxalic acid is converted into the first polymorph, hydrogen bonding occurs. It is possible to generate a chain-like structure at the intermolecular level as a result of this hydrogen bonding. It is also possible that the second polymorph of this chemical is subject to hydrogen bonding. But in this particular scenario, hydrogen bonding results in an intermolecular structure that is similar to a sheet-like structure for the molecule. Because of two significant characteristics of this chemical, it is commonly used in esterification reactions. The acidic nature of oxalic acid is the first characteristic that makes it an excellent choice for esterification processes. The hydrophilic nature of oxalic acid is the second and most essential characteristic of the compound.
Preparation of Oxalic Acid
Oxalic acid can be easily made by oxidising certain carbohydrates, such as sucrose, in the presence of concentrated nitric acid solutions. Oxalic acid is formed when the carbon atoms are torn apart in pairs during the oxidation process.
Uses
- It is employed as a mordant in the dyeing process
- It is employed in the removal of rust
- It is a significant reagent in lanthanide chemistry and is utilised in many other fields as well
- It is used to polish marble statues in order to make them sparkle
- It is employed in the production of dyes and in the production of bleaches
- It is employed in the removal of food and ink stains
- It is employed in the development of photographic film
- It is used in wastewater treatment to eliminate calcium deposits that have built up over time
Health Hazards
Oxalic acid is a poisonous substance. Vomiting, diarrhoea, and severe gastrointestinal disturbance are among the hazardous symptoms that might result from ingestion. Other symptoms include kidney damage, shock, convulsions, and coma. Cardiovascular collapse has the potential to result in death. Oxalic acid is a skin irritant that can cause irritation of the eyes, mucous membranes, and skin. Kidney injury may occur as a result of inhalation or ingestion.
Oxalic acid is a poisonous substance. Renal damage, shock, and convulsions are some of the hazardous side effects. The toxicity is caused by oxalic acid reacting with the calcium in the tissues to generate calcium oxalate, which causes the calcium potassium ratio to be thrown out of balance. The accumulation of oxalates in the kidney tubules has the potential to cause kidney injury.
Headaches, dizziness, nausea, vomiting, convulsions, coma, and even death can occur as a result of oxalic acid overdose in some cases. Excessive or repeated exposure to the chemical can cause a skin rash, pain, redness, blisters, and slow-healing ulcers, among other symptoms.
Conclusion
Oxalic acid is found in leafy greens, lentils, and the majority of other plant foods. You acquire it through your diet because it’s a naturally occurring molecule. It is also excreted by the body as waste. Oxalates-rich foods also contain a variety of additional nutrients that your body requires for optimal health.
When you ingest meals that contain oxalate, the chemical attaches itself to minerals, causing the minerals to combine to form additional minerals. Iron oxalate and calcium oxalate are examples of such compounds.
Diets low in oxalates can be beneficial if you have a high risk of kidney stone production as a result of high levels of oxalates in your body. Broccoli, cheese, and canned fish with bones are examples of such foods. They also contain significant amounts of calcium.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out



