Introduction
The heat evolved at constant volume, and heat change at constant pressure is measured using a calorimeter. The process is called Calorimetry. Bomb Calorimeter is used to measure the ∆U, while a coffee calorimeter is used to measure ∆H.
Calorimetry also helps in determining if a reaction is endothermic or exothermic. Now let us in detail understand what Calorimetry is.
What Is Calorimetry?
Calorimetry is the process of measurement of ∆U and ∆H. This process includes the use of a calorimeter. The technique includes submerging the vessel or calorimeter in a known liquid volume. The heat evolved during the process is evaluated by calculating the difference in the heat capacities of the known liquid and the calorimeter.
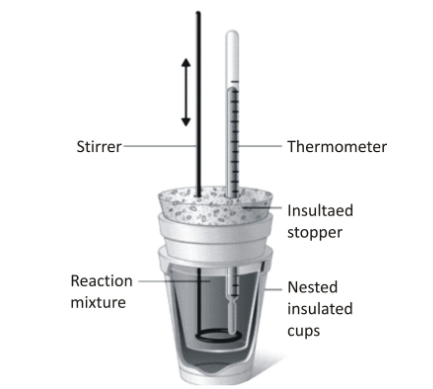
There are two ways to carry out calorimetric measurements. They are:
- Under constant-volume that is ∆U
- Under constant pressure that ∆H
Calorimetry uses thermometric methods to determine the internal energy and enthalpy. In simple words, this process measures the amount of heat transferred in a substance.
Different types of Calorimetry analysis techniques are as follows:
- Sorption Calorimetry
- Isothermal MicroCalorimetry
- Reaction Calorimetry
- Isothermal Titration Calorimetry
Before moving forward to understand the measurement process of ∆U and ∆H, let us first know in brief what enthalpy and internal energy are.
What Is Enthalpy?
Enthalpy is the heat energy that is evolved or absorbed during a chemical reaction. Enthalpy is represented by the capital letter H. On the other hand, the change in enthalpy is written as ∆H. Here the delta symbolizes the change in energy.
The unit of energy is joules or kilojoules.
In other words, enthalpy can also be defined as the sum of the internal energies of a chemical reaction. It is because the change in internal energy happens only during a chemical reaction. This change in internal energy is calculated as enthalpy change.
H = U + PV
Where,
H = Enthalpy
U = sum of all internal energies
P = pressure
V = volume
Enthalpy is the sum of internal energy and the energy required to keep a system’s volume constant at a given pressure. PV denotes the work that must be done on the environment to make room for the system.
Moreover, according to Hess’s Law, enthalpy change remains independent of the path a chemical reaction takes. The enthalpy change of a specific reaction that happens in one step is equal to the same chemical reaction that takes multiple steps to complete.
Enthalpy depends on the initial and final stages of the chemical reaction and therefore is a state function.
What Is Internal Energy?
The internal energy is equal to the sum of its kinetic and potential energy. Potential energy is the static energy that is stored in the system. On the other hand, kinetic energy is the energy that is released due to the movement of the molecules.
Internal energy is denoted by the capital letter U. The change in internal energy is represented by ∆U, where delta denotes the change.
Change in internal energy is due to two factors:
- Transfer of heat in a chemical reaction when heat is either absorbed or released.
- By doing work
Therefore internal energy change can be written as:
∆U = q + w
Where,
q = transfer of heat
w = work done on or by the system
∆U Measurement
In a chemical reaction, the heat evolved at constant volume or internal energy change is measured using a bomb calorimeter.
Bomb calorimeter consists of an inner vessel that is known as the bomb. The covers are made of strong steel and tightly fitted with metal screws and lids. The inner vessel is surrounded by a bigger insulated vessel that contains the water. It also has a thermometer and a stirrer suspended in the water.
A known amount of a combustible substance is taken in a platinum cup. The platinum cup has wires attached to it to initiate the combustion process. The bomb is then pressurized with excess oxygen after tightly sealing it. Now bomb is immersed into the bigger vessel filled with water.
Then the current is passed through the filament attached to the platinum cup in the bomb to ignite the combustion process. As the combustion takes place, the temperature of the water rises. The temperature rise is measured using the Beckman thermometer. Since the volume does not change, hence the heat change is equal to the heat of combustion.
Now the amount of heat evolved ΔUc during the procedure is equal to the heat absorbed by the water and the calorimeter.
To calculate the heat absorbed by the calorimeter:
q1 = k.ΔT
where,
k = calorimeter constant which is equal to mc Cc ( Here mc is mass of the calorimeter and Cc is heat capacity of calorimeter)
Calorimeter constant can be evaluated by burning a known amount of any standard sample for which the heat of combustion is also known.
To calculate the heat absorbed by the water:
q2 = mw Cw ΔT
where,
mw = molar mass of water
Cw = molar heat capacity of water
(4.184 kJ K-1 mol-1)
Therefore, to calculate the heat evolved during the process:
ΔUc = q1 + q2
=k.ΔT + mw Cw ΔT
=(k+mw Cw)ΔT
∆H Measurement
A coffee cup calorimeter is used to measure the heat evolved at constant pressure, that is ∆H. A Styrofoam cup is used in a coffee cup calorimeter.
It works as a good adiabatic wall, preventing the transfer of heat generated during the reaction to the environment. The water within the cup absorbs all of the heat energy. We can utilize this approach for reactions that do not significantly change the volume.
The change in the temperature of the water is equal to the heat absorbed or evolved during the chemical process. It is calculated from the following equation:
q = mw Cw ΔT
where,
mw = molar mass of water
Cw = molar heat capacity of water (4.184 kJ K-1 mol-1)
Take an example to calculate the enthalpy of combustion ethylene at 300K.
Take the heat of combustion at constant volume ∆U = -1406 kJ
Combustion reaction:
C2H4 (g) + 3O2(g) → 2CO2 (g)+2H2O(l)
ΔU = −1406 kJ
∆n = np(g) – nr(g)
∆n = 2 – 4 = -2
∆H = ∆U + ∆ngRT
∆H = -1406 + ((-2) x 8.314 × 10-3 × 300 )
∆H = -1410.9 kJ
Conclusion
Calorimetry and the measurement of ∆U and ∆H is a significant topic in thermodynamics. You need to understand every equation and process of measurement of enthalpy and internal energy to do well in the exam.
.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





