Explanation
Chemical Equilibrium
In a chemical reaction, the state where both reactants and products are present in constant concentrations, with no tendency to change in time, is called a chemical equilibrium state. This state occurs when the forward and reverse reactions happen at the same rate. The reaction rates of both the backward and forward reactions are equal and opposite. Consequently, there are no net changes in the concentrations of the reactants and products.
For example, a bottle of fizzy drink has CO2 dissolved in liquid and the surroundings. However, no transfer of CO2 occurs until and unless the environment is hampered.
There are two types of equilibrium that are physical and chemical where chemical equilibrium is dynamic and physical equilibrium is static.
1. Static equilibrium
When The forces of action and reaction cancel each other, and no change occurs, it is called a static equilibrium state—for example, a book lying on the table.
2. Dynamic equilibrium
Both action and reaction are continuously happening in equal amounts and opposite directions. It is called a dynamic equilibrium state. For example, a man going upwards in an escalator that’s going downwards.
Characteristics of chemical equilibrium
- Temperature of the reaction reaches a stable value.
- Pressure of the reaction reaches a stable value.
- Concentrations of all the reactants and products reach stable values.
- Chemical Equilibrium is always dynamic
- Equilibrium can be achieved from both sides.
- Equilibrium can be achieved only in a closed system.
- A catalyst can not alter the equilibrium state.
Chemical reactions
A process where two or more substances mix together and form new substances. They are of two types
- Reversible reactions: if under particular circumstances, forward and reverse reactions happen at the same time or the original reactant can be obtained back from the created product, then the reaction is called a reversible reaction.
- Irreversible reactions: when a reaction occurs in only one direction and the products cannot give back the reactants under any circumstances, it is called an irreversible reaction.
Law of chemical equilibrium
The law of chemical equilibrium shows a relationship between the concentrations of reactants and products. It states that the equilibrium constant is the ratio of the product of product side concentrations to the product of reactants side concentration. Accordingly, each term should be raised to the power of their stoichiometric coefficient in a balanced chemical equation.
Equilibrium constant
At a given temperature, the value of the equilibrium constant expresses the relationship between the concentration of products and reactants present at chemical equilibrium in a reversible chemical reaction. It is denoted by the letter K, and its formula is,

At a given temperature, it has a definite value for every reaction independent of the original concentration of reactants. It predicts the extent and direction of a given reaction.
When partial pressures are used instead of molar concentrations, the constant is called the pressure equilibrium constant. Kp represents it, and its formula is
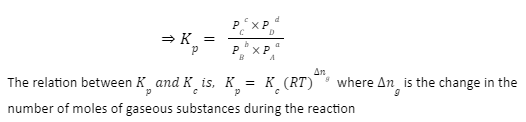
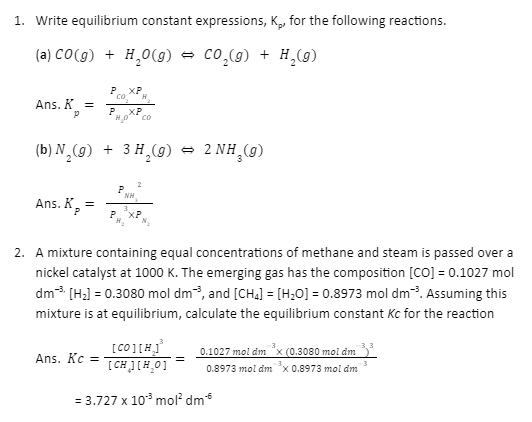
Sample problems on the law of chemical equilibrium
Applications of the law of chemical equilibrium
The law of chemical equilibrium or the law of mass action finds it used in many places. Some of the most important ones are,
- Semiconductor physics and electronics
- Social physics
- Mathematical Ecology
- Mathematical epidemiology
Conclusion
In this section, we learned about equilibrium and its types, Chemical equilibrium, and its law. We also learned about the various applications of chemical equilibrium. Finally, we did some sample problems on chemical equilibrium.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





