When halogens react with one another, interhalogen compounds are formed. Some specific halogens perform numerous regulatory roles in human beings, while others are not fundamental. Halogens tend to be nonmetals. At room temperature, chlorine and fluorine, in many instances, are fumes, bromine is fluid and Iodine, that’ll be astatine, can be solid.
Halogens are reactive; the reactivity decreases from fluorine to astatine. Halogens do not occur to the type that is nature that is elemental, and Astatine isotopes are radioactive with brief half-lives.
Most of the halogens in response with hydrogen sort compounds which may be binary substances, are called the hydrogen halides: hydrogen fluoride (HF), hydrogen chloride (HCl), hydrogen bromide (HBr), hydrogen iodide (HI), and hydrogen astatide (HAT).
Except for HF, the rest of the halogens form powerful chemical acids. However, hydrofluoric acid possesses disastrous properties in the tissue of animals, including humans.
Considering its halogen friends, hydrogen astatide must also behave as a strong acid (hydrostatic acid). But, it is a mild acid and often forgotten to be counted among the corrosive hydrohalic acids.
Metal Halides
Metal Halides are compounds between halogen and metals. Some are covalently relationships, and several are ionic. Covalently bonded metal ions may develop frameworks being polymeric. Metal Halides are created when all halogens react with the material. It is known in the equation below.
2M + nX2 2MXn
Silver Chloride: The precipitate is formed if the solution of silver nitrate is added to the chloride solution.
AgNO3(aq)+NaCl(aq)⟶AgCl(s)+NaNO3(aq)
Alkyl halide
Alkyl halides, RX, where R is an alkyl group and X is a halogen F, Cl, Br, or I)
According to the number of carbon attached to the parent carbon to which the halogen is also attached, it is classified into three types: Primary, Secondary, or Tertiary.
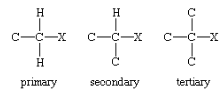
The general reaction to prepare alkyl halide is:
Alkane + Halogen (X) → Alkyl halide (R-X) + Halogen acid (H-X)
This reaction can also be said to convert alkanes into alkyl halide.
Three main methods prepare alkyl halides:
- The first is the reaction of an alkane with diatomic chlorine, as in the following synthesis of chloroethane:
C2H6 + Cl2 C2H5Cl + HCl
Similarly, many alkyl halides are formed, such as Chloromethane, Chloroethane, Bromoethane, Bromoethane.
- The second method is the addition of a hydrogen halide to an alkene; e.g.,
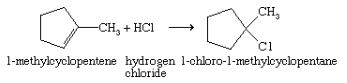
- The third method is free-radical halogenation of an alkane; e.g.,
CH3CH2CH2CH3 Cl2/UV light HCl+ CH3CH2CH2CH2Cl
Vicinal dihalides have halogens on either side of the carbon and are prepared by reacting a halogen and an alkene. For eg: 1,2-dichloroethane (ethylene dichloride).
Aryl halides
Aryl halides are formed when a benzene ring reacts with a halogen.
Below are some aryl halide reactions:
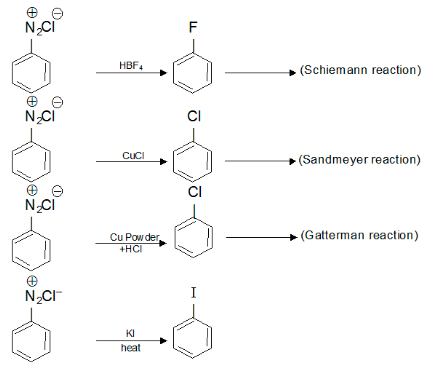
Conclusion
Halogens are elements that belong in group 17 of the periodic table, and these elements are present both in nature and are produced synthetically. Halogen compounds occur as hydrogen halide, metal halides, polyhalogenated, organohalogen compounds, and the alkyl halide, vinyl halide, and aryl halide. Fluorine is the most famous halogen, with the highest reactivity and smallest size. Amongst the halogen compounds, the most ignored and the most radioactive is astatine—chlorine, bromine and Iodine fall in the middle.
Halogen atoms are lipophilic and less water-soluble. Halogens are not solely confined to organic chemistry, they can be found around us, and we use them daily.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





