Introduction to Halides
Halides are compounds whose one part is made of halogens X (F, Cl, Br, I), and the other part constitutes an element or a radical that is less electronegative than the halogen.
The bonds between the halogen and the other element can either be covalent or ionic (in metal halides).
There are 13 subcategories of halides. Here we will study organohalides in detail. They are further classified based on the number of halogen atoms, into aliphatic and aromatic, and the basis of hybridisation of the carbon present (into alkyl halides, allylic halides, benzylic halides, vinylic halides, and aryl halides).
Compounds with sp3 hybridised C-X bond
Haloalkanes (alkyl halides)
The halogen atom (F, Cl, Br, I) is bonded to an alkyl group in haloalkanes. It means that one of the hydrogens of an alkane is replaced by a halogen atom.
Their general formula is CnH2n+1X, and they form a homologous series.
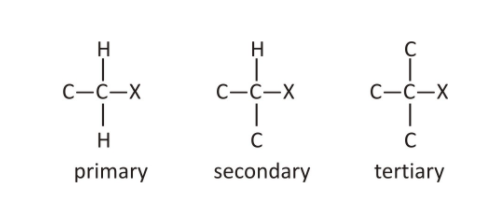
Primary alkyl halides
If the primary carbon has a halogen group attached, the alkyl halide is known as primary or 1° alkyl halides.
Examples – CH3 – CH2 – Cl, CH3 – CH2 – CH2 – CH2 – Br
Secondary alkyl halides
In secondary alkyl halides, the carbon is attached to a secondary carbon. They are also known as 2° alkyl halides.
Example – CH3– CH (CH3) – Cl
Tertiary alkyl halides
The halogen atom in tertiary alkyl halide is attached to a 3° carbon.
Example – CH3– C (CH3)2– Br
Allylic halides
The allylic carbon atom is an sp3 hybridised C atom attached to an sp2 hybridised C atom or a carbon atom with a double bond. If a halogen is attached to this allylic carbon, the compound is known as an allylic halide.
Examples – CH3 – CH2 – CH2 – CH = CH – CH2 – Cl
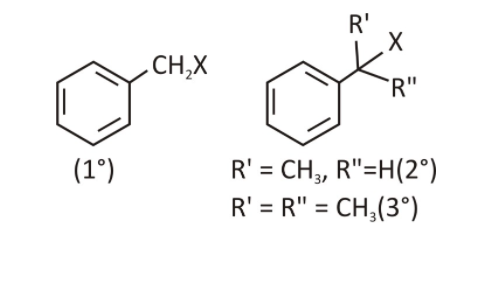
Benzylic halides
In these compounds, the halogen group is attached to an sp3 hybridised C atom bonded to an aromatic (benzene) ring.
Compounds with sp3 hybridised C-X bond
Vinylic Halides
They have a general formula CH2=CHX, i.e., the halogen is attached to that doubly-bonded C atom which is sp2 hybridised (C = C).
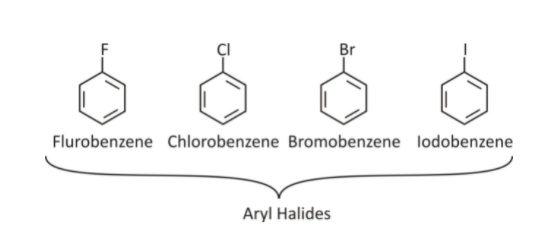
Aryl Halides (haloarenes)
These are aromatic compounds in which the halogen atom is directly attached to the sp2 hybridised C of a benzene ring.
Methods of preparation of haloalkanes
From alcohols
- By the reaction of halogen acids (HCl, HBr, HI)
- HCl – ZnCl2 is used as a catalyst
CH3 – CH2 – OH + HCl → CH3 – CH2 – Cl + H2O
CH3 – CH – OH + HCl → CH3 – CH – Cl + H2O
| |
CH3 CH3
- HBr – In situ formation of HBr includes the reaction of potassium bromide with sulphuric acid.
KBr + H2SO4 → HBr + KHSO4
CH3 – CH2 – OH + HBr → CH3 – CH2 – Br + H2O
CH3 – CH – OH + HBr → CH3 – CH – Br + H2O
| |
CH3 CH3
- HI – In situ formation of HI includes the reaction of potassium iodide with phosphoric acid.
KI + H3PO4 → HI + KH2PO4
CH3 – CH2 – OH + HI → CH3 – CH2 – I + H2O
CH3 – CH – OH + HI → CH3 – CH – I + H2O
| |
CH3 CH3
Tertiary haloalkanes are the most reactive because they form the most stable carbocation. The order of reactivity is 3° > 2° > 1°. The order of reaction for acids follow the order HI > HBr > HCl > HF. This is because the H-I bond is the weakest.
- By the reaction of PCl5, PCl3, and SOCl2
Haloalkanes can be obtained by phosphorus halides and thionyl chloride by the following reactions.
CH3 – CH2 – OH + PCl5 → CH3 – CH2 – Cl + POCl3
3 CH3 – CH2 – OH + 3 PCl3 → 3 CH3 – CH2 – Cl + H3PO3
CH3 – CH2 – OH + SOCl2 → CH3 – CH2 – Cl + SO2 + HCl
From alkenes and alkynes
- By the addition of halogen acids (HCl, HBr, HI)
- CH3 – CH = CH2 + HCl → CH3 – CH – CH3
|
Cl
The Markownikoff rule is applied here. It states that the negative part of the reagent is added to that double-bonded C atom which has fewer hydrogen atoms.
- CH3 – CH = CH2 + HBr → CH3 – CH – CH3
|
Br
This reaction is carried out in the presence of peroxide. The Kharasch effect/ peroxide effect/ anti-Markownikoff rule is followed here.
- By reaction with halogens
- In the presence of CCl4 or dark
CH2 = CH2 + Br2 → CH2 – CH2
| |
Br Br
- In the presence of sunlight/ultraviolet rays
CH3 – CH = CH2 + Br2 → CH2 – CH = CH2 + H2O
|
Br
From alkanes (halogenation)
- Chlorination
Chlorine reacts until all the hydrogen atoms are replaced.
- Bromination
CH4 + Br2 → CH3Br + HBr
- Iodination
It is carried out in the presence of an oxidising agent such as HIO3 or HNO3.
CH4 + I2 → CH3I + HI
Finklestein reaction (halogen substitution)
CH3Br + NaI → CH3I + NaBr
CH3Cl + NaI → CH3I + NaCl
CH3F + NaI → CH3I + NaF
- Fluorination
Direct fluorine is not added to alkanes as the reaction is explosive. Therefore, a halogen substitution method is used.
Swarts reaction
CH3Br + AgF → CH3F + AgBr
2 CH3Br + Hg2F2 → 2 CH3F + Hg2Br2
3 CH3Br + SbF3 → 3 CH3F + SbBr3
Conclusion
Halides are halogen-containing compounds. Organic compounds with a halogen group attached to them are known as organohalides. Haloalkanes are aliphatic, and aryl halides (haloarenes) are aromatic. Allylic halides and benzylic halides are sp3 hybridised, while aryl and vinylic halides are sp2 hybridised. Alcohols can prepare Haloalkanes by adding halogen acids, phosphorus halides and thionyl chloride.
To obtain alkyl halides from alkenes and alkynes, halogen halides are used. Due to the presence or absence of peroxide, Markownikoff or anti-Markownikoff rule is applied. In the presence of sunlight, halogens attack allylic carbon.
The direct addition of halogens to an alkane is called halogenation. Fluro Halides are prepared by the Swarts reaction or halogen exchange method.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





