When you study for national-level examinations like IIT JEE Mains or Advance, you cannot afford to miss even a single topic as it might haunt you while writing the paper. And if it’s one of the most crucial topics, there’s absolutely no escape. In the list of important topics to crack IIT JEE Mains, one such topic is General trends in properties of the first-row transition elements that cover a vast part of their paper. Thus, to better understand this topic, Unacademy has decided to help you throughout the process.
No matter if you’re ready to appear in the exam or not, Unacademy has curated the best study material for you all. Today, in this article, we will be explaining your transition elements and other crucial topics related to the same so make sure you stick with us till the end. Without any further ado, let’s dive into it.
What are Transition Elements?
Transition elements, commonly known as transition metals, are those elements that have partially filled the f and d subshells. As per the IUPAC, transition elements have a partially electron-filled d subshell of an element which can give rise to cations that are stable with incomplete d sub-shell.
Primarily, the transition elements are those elements that are in the d-block of the modern periodic table. However, the elements in the f block can also be termed as transition metals. Although the f block elements have partially filled orbitals, these are known as inner transition metals or inner transition elements.
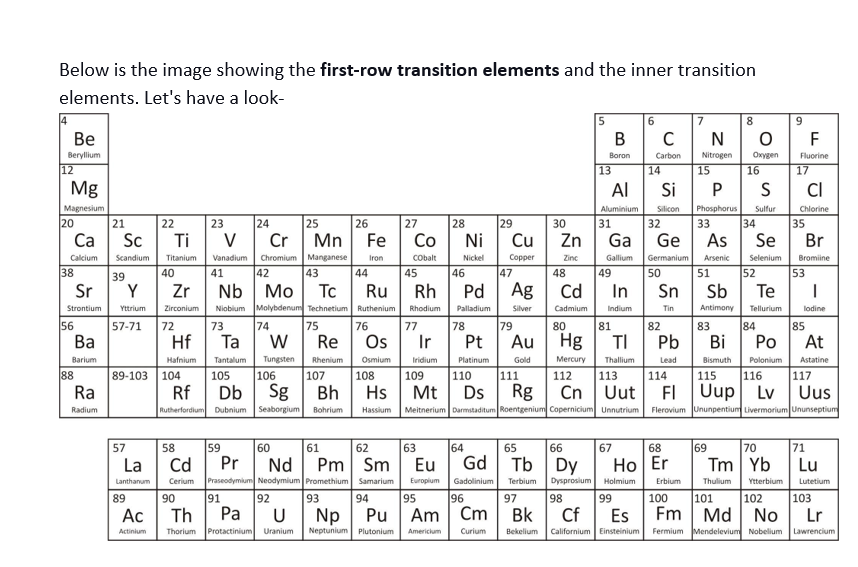
Below is the image showing the first-row transition elements and the inner transition elements. Let’s have a look-
Because of the electronic configuration (n-1)d10 ns2, specific elements, including cadmium, zinc, and mercury, are not considered transition elements.
Transition Elements Electronic Configuration
Below, we have listed the first two rows of transition elements with their atomic number, and electronic configuration in a tabulated form. If seen closely, it is pretty evident that in some of the elements, electronic configuration corresponds to (n-1)d5 ns1 or (n-1)d10 ns1. The reason behind this is the stability generated by the complete or half-filled electron orbitals.
Transition Elements | Atomic Number | Electronic Configuration |
Sc | 21 | [Ar] 3d1 4s2 |
Ti | 22 | [Ar] 3d2 4s2 |
V | 23 | [Ar] 3d3 4s2 |
Cr | 24 | [Ar] 3d5 4s1 |
Mn | 25 | [Ar] 3d5 4s2 |
Fe | 26 | [Ar] 3d6 4s2 |
Co | 27 | [Ar] 3d7 4s2 |
Ni | 28 | [Ar] 3d8 4s2 |
Cu | 29 | [Ar] 3d10 4s1 |
Zn | 30 | [Ar] 3d10 4s2 |
Y | 39 | [Kr] 4d1 5s2 |
Zr | 40 | [Kr] 4d2 5s2 |
Nb | 41 | [Kr] 4d4 5s1 |
Mo | 42 | [Kr] 4d5 5s1 |
Tc | 43 | [Kr] 4d5 5s2 |
Ru | 44 | [Kr] 4d7 5s1 |
Rh | 45 | [Kr] 4d8 5s1 |
Pd | 46 | [Kr] 4d10 |
Ag | 47 | [Kr] 4d10 5s1 |
Cd | 48 | [Kr] 4d10 5s2 |
Like chromium, many transition elements have not followed the Aufbau Principle. The reason behind this is the relatively low energy gap between 3d and 4s orbitals, and the 4d and 5s orbitals.
Transition Elements General Properties
As mentioned earlier, the electronic configuration of elements like cadmium, zinc, and mercury differs from that of all other transition metals; these are not considered one of those. However, the left-out elements have similar properties and electronic configuration, which can be observed in the above table mentioned. The general properties of the first row transition elements are listed below; let’s have a look-
- These elements form coloured ions and compounds. The colour formed is explained by the d-d electrons transition.
- These elements have a relatively low energy gap in several oxidation states.
- These elements form several paramagnetic compounds because of d orbital unpaired electrons.
- A large number of ligands can bind themselves to these elements. Because of this, several stable complexes are developed by transition metals or transition elements.
- Transition elements enjoy a larger ratio of radius charge.
- The transition elements are way harder and have a comparatively higher density than other elements.
- The transition elements have high boiling and melting points due to the interference of the delocalised d electrons.
- Because of this interference of metallic bonding in d electrons develops transition elements into great conductors of electricity.
Many of the first row transition elements enjoy catalytic properties, which play a significant role in producing certain chemicals. One of its examples is iron which is used to prepare ammonia.
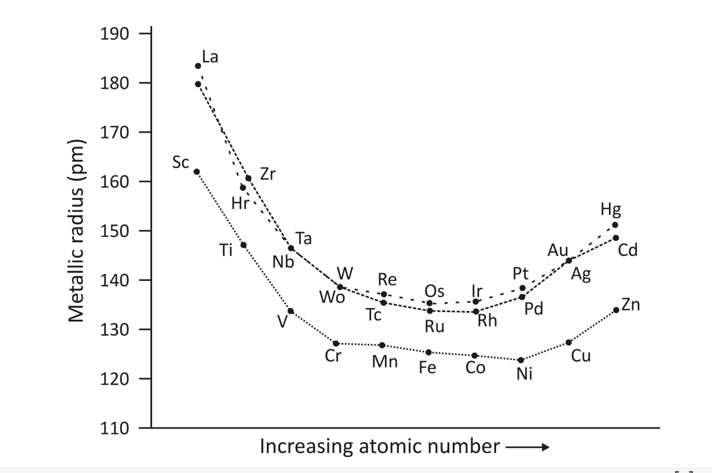
Atomic Ionic Radii
Due to the poor shielding, the ionic and atomic radii decrease from group 3 to group 6. Elements that are placed in group 7 to group 10 have similar atomic radii and those placed in group 11 and group 12 have larger radii. This happens because the nuclear charge is greatly balanced due to electron-electron repulsions.
When you look at the above graph, it can be observed that atomic and ionic radii increase while traversing down the group.
Ionization Enthalpy
The Ionization enthalpy can be defined as the energy consumed by the gaseous atom to expel an electron in its gaseous form. Every time an electron is withdrawn from an atom, a considerable amount of energy is consumed to lose it. This is known as Ionization enthalpy. Ionization falls from top to bottom in the periodic table, whereas it rises from left to right. Ionization enthalpies of transition elements are generally greater than those of the s- block elements.
The ionization energy for every ion is calculated in the periodic formula. It is first important to know the calculation behind measuring the energy required to lose electrons to calculate the ionization energy. Ionization energy equation can be written as- X → X+ + e-
Every time an electron is withdrawn from a molecule or an atom, a considerable amount of energy is needed to eliminate the remaining electrons from the same molecule or atom. Therefore, the change in the equation occurs. Every time there’s a change in the amount of energy needed to remove an electron, the equation itself changes.
Conclusion
While this is about the general trends in properties of the first-row transition elements and other vital topics related to the same, now, you can continue your learning practice by going through our other articles on different important topics, as Unacdemy covered it all for you. Unacademy hosts a range of valuable study material to help you score high marks in your upcoming board exams or further entrance exams.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out