The transformation of an organic compound into another using anhydrous aluminium chloride as the catalyst: e.g. An aromatic hydrocarbon is alkylated with an alkyl halide to form a hydrocarbon (ethylbenzene). A ketone is produced by reacting an aromatic hydrocarbon (as benzophenone) with an acyl chloride or acid anhydride.
What is Friedel-Crafts Reaction?
Friedel-Crafts reactions act as an electrophilic aromatic substitution of an aromatic compound. Chemists Charles Friedel, along with James Crafts, developed this popular reaction. The aromatic compound undergoes electrophilic substitution in the Friedel-Crafts reaction. Lewis acids like anhydrous aluminium chloride substitute the hydrogen atom in benzene with an electrophile. Alkylation and Friedel-Crafts acylation reactions are the two Friedel-Crafts reactions.
Friedel-Crafts Acylation Reaction Meaning
An acylation of aromatic rings occurs in the Friedel-Crafts acylation reaction. The most common agent for this reaction is acyl chloride. Carboxylic acids and acid anhydrides are also feasible. An aluminium trichloride catalyst is common in Lewis acids. In contrast to the Friedel-Crafts alkylation Reaction, the resulted product ketone constitutes a relatively stable complex alongside Lewis acids, for example, AlCl3, requiring only a small amount of catalyst; in contrast to Friedel-Crafts alkylation, the catalyst must be constantly regenerated.
Friedel-Crafts alkylation has the same reaction conditions. In comparison with alkylation, this reaction also has many advantages. Ketone products are always less reactive than the original molecules because of the electron-withdrawing nature of any carbonyl group; therefore, no multiple acylations occur. In addition, there are negligible carbocation rearrangements since positive charges present in the oxygen stabilise the acylium ion.
For the Friedel-Craft reaction on acylation to be successful, acyl chloride reagents must be stable. To isolate, for example, formyl chloride is overly unstable. Therefore, formyl chloride must be synthesised in situ to make benzaldehyde using the given Friedel-Crafts pathway. An aluminium/cuprous chloride catalyst catalyses the Gattermann-Koch reaction, which involves ministering benzene with hydrogen chloride and carbon monoxide under elevated pressure. By alternative methods, e.g., oxidation, industries produce normal ketones that can be acquired by the Friedel-Crafts reaction for acylation.
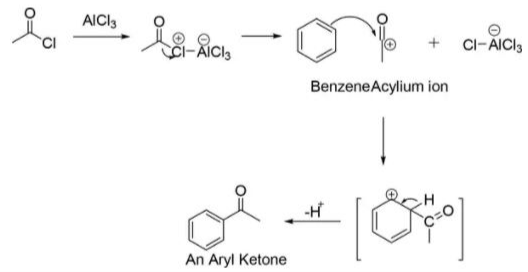
Friedel-Crafts Acylation Reaction Mechanism
A centre of acylium is generated in the reaction. Regeneration of the AlCl3 catalyst occurs when arenium ions are deprotonated by AlCl4-. Instead of a fully catalytic alkylation, the generated ketone forms a type of complex along with a Lewis acid (strong) aluminium trichloride. Under the reaction conditions, this complex typically forms irreversibly. Therefore, AlCl3 is needed in stoichiometric quantities. An aqueous workup destroys the complex to yield the expected ketone. In addition to Germanic acylation, the Friedel-Crafts acylation reaction can be performed using catalytic quantities of given milder Lewis acids (for example, Zn(II) Zinc salts) or Bronsted acids catalyst that utilises carboxylic acid or the anhydride as an acylation agent. If desired, one can reduce the resulting ketone through Clemmensen reduction or Wolff-Kishner reduction to the corresponding alkane substituent. The net outcome is equivalent to the Friedel-Crafts alkylation. Aside from that, modification is preposterous.
Rearrangements in the Friedel-Crafts Acylation Reaction
Unlike Friedel-Crafts alkylations, the Friedel-Crafts acylation reaction does not undergo rearrangement. Due to the carbocation rearrangement in Friedel-Crafts, alkylation allows for the use of the Friedel-Crafts acylation reaction to achieve products that are otherwise difficult to obtain. To make propyl benzene, we could, for instance, use this reaction instead of Friedel-Crafts’ alkylation reaction with AlCl3 and 1-propyl chloride. To begin, benzene and propionyl chloride need to undergo a Friedel-Crafts acylation reaction, perhaps catalysed by AlCl3. Ethyl phenyl ketone is the result. The next step will be to reduce the ketone to an alkane, which can be accomplished in several ways. 1-Propylbenzene is the result.
Limitations to the Friedel-Crafts Acylation Reaction
While the Friedel-Crafts acylation reaction overcomes a few limitations of the regiven alkylation reaction (for example, polyalkylation and carbocation rearrangement), it still has a few limitations.
- During the acylation reaction, ketones are exclusively produced. As a result, formyl chloride with the formula (H(C=O)Cl) reacts with these conditions, releasing carbon dioxide and hydrochloric acid.
- A less reactive aromatic mixture cannot be included in the given reaction. This reaction cannot be performed with aryl amines as they form a highly unreactive complex with Lewis acid’s catalyst.
- Depending on the chemical reaction, nitrogen and oxygen can be acylated with amines and alcohol.
Conclusion
The Friedel-Crafts reaction’s importance is that it plays an integral role in preparing chemicals, intermediates, and fine chemicals in the industrial world. During Friedel-Crafts acylation, reactive acylations are thought to play a key role. Due to the reactivity of the Lewis Acid, these reactions are extremely exothermic. There are two different substrates and two different catalysts, so you will perform two different Friedel-Crafts reactions.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




