When a chemical process reaches equilibrium, the equilibrium constant (often indicated by the symbol K) offers information on the relationship between the products and reactants in the reaction. For example, the equilibrium constant of concentration (denoted by Kc) of a chemical reaction at equilibrium can be defined as the ratio of the concentration of products to the concentration of reactants, each raised to the stoichiometric coefficients of the other reactants. Remember that there are multiple different types of equilibrium constants, each of which provides relationships between the products and reactants of equilibrium processes in terms of different units of measurement.
The equilibrium constant for a chemical reaction can be described as the ratio between the amount of reactant and the amount of product, which is used to identify the chemical behaviour of the reaction.
Rate of forward reaction equals Rate of backward reaction when the system is in equilibrium.
![]() During a specific temperature range, the rate constants are always the same. As a general rule, the relationship between the rate constants for forward reaction and backward reaction should be constant, and this is referred to as an equilibrium constant (Keq).
During a specific temperature range, the rate constants are always the same. As a general rule, the relationship between the rate constants for forward reaction and backward reaction should be constant, and this is referred to as an equilibrium constant (Keq).
Equilibrium constant calculation
A constant, K, can be derived for an equilibrium equation aA + bB ⇌cC + dD using the formula K = [C]c[D]d / [A]a[B]b, where K is a constant. For example, the equilibrium equation is aA + bB = cC + dD. It is necessary to place all of the products’ concentrations in the numerator, while the reactants’ concentrations are placed in the denominator; each component is then raised to the power of its individual coefficient. It is equal to one-half the rate constant of the forward reaction divided by the rate constant of the reverse reaction to obtain the equilibrium constant.
The law of mass action is the name given to this relationship, which is also known as the equilibrium constant. Firstly, the law asserts that the rate of a chemical reaction is directly proportional to the concentrations of both reactants and products present in the reaction. Also stated, and maybe more importantly for our purposes right now, is that the ratio of reactant to product concentrations is constant for a reaction at equilibrium, which is a key concept in chemical engineering. This constant is referred to as the equilibrium constant, abbreviated K.
The relationship K = [C]c[D]d/ [A]a[B]b is particularly noteworthy because it is directly equivalent to K = kforward / kreverse, where kforward is the rate constant for the forward reaction and kreverse is the rate constant for the reverse reaction:
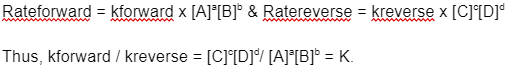
Additionally, this equation explains why K represents the extent of the reaction. It follows that the numerator, which represents the concentration of products, is bigger if K > 1. If K is less than 1, the denominator, which is represented by the concentration of reactants, is larger.
Equilibrium constant Kc and Kp
Equilibrium constant Kc
It is expressed as a ratio between the concentrations of products and reactants, and it is denoted by the symbol Kc. It is necessary to express Kc in terms of their molar concentrations in order to calculate it.

[A], [B], [C], and [D] represent concentrations of A and B reactants, as well as concentrations of C and D products. The stoichiometric coefficients of each reactant and product in the chemical equation are represented by the exponents “a,” “b,” “c,” and “d.” In the expression for Kc, the concentrations of reactants and products are increased to powers equal to their stoichiometric coefficients, which is the same as the expression for K.
Equilibrium constant kp
Kp is the equilibrium constant, and it is expressed as a ratio between the pressures of the products and the pressures of the reactants. In the case of gaseous reaction mixtures, this equilibrium constant is appropriate. The partial pressures of gaseous components in the reaction mixture determine the value of Kp.

The partial pressure is denoted by the letter “p.” As a result, pP, pQ, pR, and pS are the partial pressures of the P, Q, R, and S gas components, respectively. The stoichiometric coefficients of each reactant and product in the chemical equation are represented by the exponents “p,” “q,” “r,” and “s.”
Difference between Kc and Kp
The equilibrium constants Kc and Kp are denoted by the letters. When a reaction mixture reaches equilibrium, it is represented by a number that indicates the relationship between the concentrations or pressures of products and reactants in the reaction mixture. Essentially, the difference between Kc and Kp is that while Kc is the equilibrium constant that can be calculated from the terms of concentration, Kp is the equilibrium constant that can be calculated from the terms of pressure.
In the case of reversible reactions, this is the equilibrium constant that is given. It is the equilibrium constant Kc that is expressed as a ratio between the concentrations of products and reactants, and it is the equilibrium constant Kp that expresses itself as a ratio between the pressure of the products and reactants.
Conclusion
Chemical equilibrium is the state of a chemical reaction in which the forward reaction rate and the reverse reaction rate are both equal. As a result of this equilibrium, the concentrations of the reactants and products remain constant throughout the reaction. However, just because concentrations are not changing does not imply that all chemical reactions have come to a complete halt. On the contrary, chemical equilibrium is a dynamic condition in which reactants are continuously changed into products, but at a rate that is precisely equal to the rate at which products are turned back into reactants.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




