Catalysis is a phenomenon in which the rate of a reaction is sped up or slowed down by the use of a catalyst (the catalyst does not participate in the reaction; its concentration and composition remain unchanged). A catalyst is a substance that is used to influence the rate of a reaction. Enzymes are a type of catalyst that helps to speed up and facilitate a variety of important metabolic events in plants and animals. Enzyme catalysis is a type of catalysis in which enzymes function as a catalyst. Enzymes are nitrogen-containing complex molecules. These substances are produced spontaneously in the bodies of animals and plants. When dissolved in water, enzymes have a high molecular mass and form a heterogeneous mixture. These proteins have a high efficiency and are responsible for a variety of processes in living beings’ bodies.
Characteristics of enzyme catalysis:
- A single enzyme catalyst molecule can convert up to a million molecules of reactant every second. As a result, enzyme catalysts are believed to be extremely effective.
- These biological catalysts are specific to specific types of reactions, hence they can’t be employed in multiple processes.
- At its optimum temperature, a catalyst’s effectiveness is at its peak. The biological catalysts’ activity decreases on either side of the optimal temperature.
- The pH of a solution has an impact on biochemical catalysis. A catalyst performs best when the pH is between 5-7.
- In the presence of a coenzyme or activator such as Na+ or Co2+, enzyme activity normally increases. Due to the presence of a weak link between the enzyme and a metal ion, the reaction rate accelerates.
Mechanism of enzyme catalyst:
Enzymes have a lot of cavities on their exterior surface. These cavities have groups like -COOH, -SH, and so on. The active centre of a biological particle is defined as these centres. The substrate, which is charged in the opposite direction as the enzyme, fits into the cavities like a key into a lock. The complex produced decomposes to give the products due to the presence of active groups.
As a result, there are two steps:
Step 1: The enzyme and the reactant are combined.
E + R ER
Step 2: The complicated molecule is disintegrated to produce the final product.
E R E + R
Conclusion
Enzymes have biological as well as chemical properties. Their sequences and architectures define their function in all living creatures’ genomes and proteomes, and their ability to catalyse chemical reactions expands their biological function to metabolic pathways and networks. Many enzymes are promiscuous, performing many reactions, and their reaction profiles can alter as the protein sequence changes. To capture the core of enzyme chemistry in a functional categorization, bond modifications and reaction centres must be combined with structural information about substrates, products, and processes. The development of tools to navigate across reaction space (e.g., EC-BLAST) has paved the way for a more accurate description of enzyme reactions, allowing for a more comprehensive understanding of biological function.
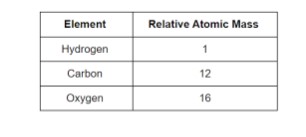
- Relative Atomic Mass
The relative atomic mass of an element is the relationship between its mass and the number of atoms it contains. The relative atomic mass scale is used to calculate the masses of different atoms.
The hydrogen atom, the lightest atom, was given a relative atomic mass of 1, and the relative atomic masses of other atoms were calculated in comparison.
- Gram Atomic Masses
The gram atomic masses of elements are their atomic masses given in gram. The atomic mass of an oxygen molecule, for example, is 16 amu.
As a result, oxygen has a gram atomic mass of 16 g.
- Molecular Mass
The number of times a material’s particle is heavier than one-twelfth the mass of a carbon atom – 12 – is the sub-atomic mass of the substance. Alternatively, the sub-atomic mass is equal to the sum of the atomic masses of the seeming plurality of particles present in a single material particle. Take, for example, water.
H has an atomic mass of one unit.
O has an atomic mass of 16 units.
Water has a subatomic mass of 2 atomic masses of H + 1 atomic mass of O.
= 2 × 1 + 16 × 1
= 18 units
Molecules’ molecular masses can be manipulated in the following way:
- Mass Spectrometry: This method is often used to determine the mass of small compounds. Monoisotopic mass is used to account for this.
- Hydrodynamic Strategy– Houwink relations are used in a hydrodynamic strategy because this approach necessitates calculation. It is sometimes referred to as a relative atomic weight determination procedure.
- Static Light Scattering: The Zimm approach is used to determine the molecular weight from the amount of light scattered.
- Mass photometry: MP is a label-free, in-solution technique of determining the molecular mass of proteins, lipids, carbohydrates, and nucleic acids at the single-molecule level. Interferometric scattered light microscopy is the basis of the technology. The mass of the molecule is linearly proportional to the contrast from scattered light caused by a single binding event at the interface between the protein solution and the glass slide. This approach may also be used to identify protein oligomerization status, characterize complex macromolecular assemblies (ribosomes, GroEL, AAV), and measure protein interactions such as protein-protein interactions.
Gram Molecular Mass
The gram sub-atomic mass is the sub-atomic mass of a material represented in grams.
Consider the following example: Oxygen has a molecular mass of 32u.
The subatomic mass of oxygen is 32 grams per gram.
- Formula Mass
Sodium chloride does not have separate molecules as component units.
For example, sodium chloride (NaCl).
Positive (sodium) and negative (chloride) entities are grouped in a three-dimensional structure in such compounds.
For example, the mass of sodium chloride (NaCl) is equal to the sum of the atomic masses of sodium (Na) and chlorine (Cl): 23.0 u + 35.5 u = 58.5 u.
Conclusion
- The mass of individual atoms and molecules is described by the atomic mass unit (u).
- The atomic mass of an element is the weight average of the masses of all its isotopes.
- The total of the masses of the atoms in a molecule is the molecular mass.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out



