When a process is carried out under constant pressure, the heat absorbed or released equals the Enthalpy change. Enthalpy is also known as “heat content,” but “enthalpy” is a more exciting and unusual word that most people prefer to use. The terms “entropy” and “enthalpy” are derived from Greek words that mean “turning” and “warming,” respectively.
Entropy is usually emphasised on the first syllable and enthalpy on the second.
Body
Bond Enthalpy
Bond enthalpy is also known as the energy of a bond, gives information about the strength and, by extension, stability of a chemical bond. One mole of a chemical’s hydrogen bond with each other, requiring a lot of energy to break it. The oxygen-hydrogen single bond, for example, has a bond enthalpy of 463 kJ/mol. This means that it takes 463 kilojoules of energy to break one mole of hydrogen-oxygen single bonds.
It should be noted that the breaking of a chemical bond is always an endothermic process (because energy must be supplied to the molecule to break the chemical bonds that constitute it). As a result, the enthalpy change associated with a chemical bond breaking is always positive (H > 0). The formation of a chemical bond, on the other hand, is almost always an endothermic process. The enthalpy change will be negative (H < 0). The quantities mean ‘bond enthalpy‘ or ‘average bond enthalpy’ can be used to express the strength of a single, specific bond in a molecule. Calculating the average value of all the bond dissociation energies of that type of bond yields the mean bond energy of a chemical bond (in a molecule). As a result, mean bond enthalpy differs from bond dissociation energy (except for diatomic molecules).
Bond enthalpy is the energy required to break one mole of chemical bonds at 298 K in the gas phase. The higher the value, the stronger the bond and the greater the amount of energy required to break the bond.’
For example, the bond dissociation energy required to break one mole of gaseous hydrogen chloride molecule into gaseous hydrogen and chlorine atoms is 432 kJ. In contrast, the bond dissociation enthalpy of gaseous HCl is +432 kJ per mol. If a molecule has multiple bonds, the bond enthalpy for each bond is calculated, and the average value is used. Methane (CH4), for example, has four C-H bonds with average bond energies of +1652 kJ and +415.5 kJ per mole of the bond.
Hydration Enthalpy
(HHyd)Hydration enthalpy is the change in enthalpy that occurs when one mole of a gaseous ion dissolves in enough water to form an infinitely dilute solution under standard conditions of 1 bar pressure (infinite dilution means a further addition of solute will not cause any heat change).
Enthalpy of hydration is defined as the amount of energy released during the dilution of one mole of gaseous ions. It can be thought of as the solvation enthalpy, with water as the solvent. Hydration enthalpy, also known as hydration energy, is always negative.
To describe a chemical reaction
M+(g) + aq → M+(aq) Enthalpy change = ∆HHyd
Water is a polar solvent because it contains both positive (H atom) and negative (O atom) poles. When an ionic compound (for example, NaCl) is dissolved in water, its solid-state structure is destroyed, and the Na+ and Cl– are separated.
They exist in solution as Na+ atoms surrounded by the opposing ends of water molecules and Cl– atoms surrounded by the positive ends of water molecules.
As new bonds are formed between the atoms, some energy is released.
The difference between M+(g) and M+(aq) hydration enthalpy or enthalpy of hydration is that in M+(aq), the ion is surrounded by water molecules, forming a weak bond.
Elements’ Hydration Enthalpy
| Ion | ꕔHHyd |
Li+ | -520 |
Na+ | -405 |
K+ | -321 |
Rb+ | -300 |
Cs+ | -277 |
F– | -506 |
Cl– | -364 |
Br– | -337 |
I– | -296 |
The magnitude of the hydration enthalpy is determined by the ion charge density. Because smaller ions have a higher charge density, they have higher hydration enthalpy values. The greater the charge density, the stronger the attraction between the ion and the water polar end. In smaller ions, this increases the value of hydration enthalpy. The alkali metals are highly hydrated, with the degree of hydration decreasing as one moves down the group.
Solubility, Enthalpy, and Hydration
The ions in a solute are held together by the coulombic force of attraction; to dissolve this solute into the solvent (in this case, water), the water molecule must overcome this strong force of attraction. Lattice enthalpy is the energy required to break this string’s force of attraction.
Most ionic compounds are insoluble in non-aqueous solutions but highly soluble in water. The ions’ interactions with the solvent determine a salt’s solubility.
As previously stated, water is a polar molecule with a partial positive charge on hydrogen and a partial negative charge on oxygen that interacts with ions to form a strong bond that releases energy.
The dissolution process can be thought of as a combination of two processes.
The first is this:
Lattice enthalpy ∆HLatt= M+(s) → M+(g) ∆
hydration is the second process,
Hydration enthalpy ∆HHyd= M+(g) + aq → M+(aq) ∆
The first process is endothermic because it involves the breaking of bonds in the solid solute. The energy released when one mole of ionic solid is converted into gaseous ions is defined as lattice enthalpy. The greater the lattice enthalpy, the greater the amount of energy required to overcome the force of attraction. Because of their higher lattice enthalpy value, some compounds are insoluble in water.
Ionization Meaning
In more technical terms, ionisation energy is defined as the minimum amount of energy that an electron in a gaseous atom or ion must absorb to escape the influence of the nucleus. It is also known as ionisation potential and is typically an endothermic process.
We can also conclude that ionisation energy provides information about the reactivity of chemical compounds. It is also useful for determining the strength of chemical bonds. It is measured in either electron volts or kJ/mol units.
Depending on how molecules are ionised, which frequently results in changes in molecular geometry, ionisation energy can be either adiabatic ionisation energy or vertical ionisation energy.
Factors Affecting Ionisation Energy
When the ionisation energy is high, it is typically more difficult to remove an electron. The attraction forces are also governed by a number of factors.
- An electron is pulled towards a positively charged nucleus by a force.
- When an electron is close to the nucleus, the attraction is stronger than when the electron is further away.
- The attraction forces are reduced as the number of electrons between the outer level and the nucleus increases.
- When two electrons are in the same orbital, they are repelled in some way. This now causes disturbances in the nucleus’s attraction. In essence, because paired electrons can be easily removed, their ionisation energy will be lower.
Ionisation
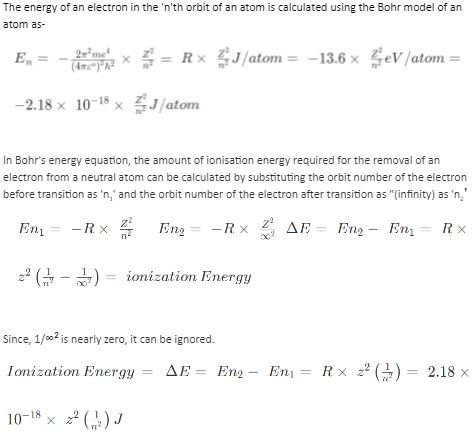
Ionisation is the removal of an electron from an orbit to the outside of the atom. The difference in energy between the energy of the electron in the initial orbit and the energy of the electron outside the atom is determined by the electrons’ orbital energy.
The energy of an electron in the ‘n’th orbit of an atom is calculated using the Bohr model of an atom as-
Ionization examples
The quantity or amount of energy required to expel an electron from the gaseous form of an atom or molecule is referred to as ionisation energy. As electrons are removed, it becomes more difficult to remove another because the atom’s charge has changed, and the electron is more attracted to remain with the atom. As a result, different ionisation energies may be required under different conditions.
Example of Sodium Ionisation Energy
Consider sodium as an example of ionisation energy in action. In this ionisation energy example, Na denotes sodium, and e- denotes the electron removed from the sodium atom.
1 Ionisation level (IE1):
Na(g) → Na+(g) + e-
IE1 = 496 kJ/mol
2 Ionisation level (IE2):
Na+(g) → Na2+(g) + e–
IE2 = 4560 kJ/mol
3 Ionisation level (IE3):
Na2+(g) → Na3+(g) + e–
IE3 = 6913 kJ/mol
Examples of Ionisation Energy at Extreme Levels
The elements that require the most ionisation energy at the first level of ionisation are listed in this table.
| Atomic Number | Name | 1st Ionisation Energy Level in Electron Volts (eV) or kilojoules/moles (kJ/mol) |
| 2 | helium | 24.59 or 2372.3 |
| 10 | neon | 21.56 or 2080.7 |
| 9 | fluorine | 17.42 or 1681.0 |
| 18 | argon | 15.76 or 1520.6 |
| 7 | nitrogen | 14.53 or 1402.3 |
| 36 | krypton | 14 or 1350.8 |
| 8 | oxygen | 13.62 or 1313.9 |
| 17 | chlorine | 12.97 or 1250.3 |
| 54 | xenon | 12.13 or 1170.4 |
| 6 | carbon | 11.26 or 1086.5 |
Multiply by 96.4689 to convert eV to kJ/mol. Because of rounding, some figures differ.
Examples of Ionisation Energy: Lowest Levels
After you’ve looked at the highest levels of ionisation energy, look at examples of the lowest levels of ionisation energy.
| Atomic Number | Name | 1st Ionisation Energy Level in Electron Volts (eV) or kilojoules/moles (kJ/mol) |
| 55 | cesium | 3.89 or 375.7 |
| 87 | francium | 4.07 or 380 |
| 37 | rubidium | 4.18 or 403.0 |
| 19 | potassium | 4.34 or 418.8 |
| 103 | lawrencium | 4.9 or 470 |
| 89 | actinium | 5.17 or 499 |
| 56 | barium | 5.21 or 502.9 |
| 88 | radium | 5.28 or 509.3 |
| 3 | lithium | 5.39 or 520.2 |
| 71 | lutetium | 5.43 or 523.5 |
Solution
A solution is a homogeneous mixture of two or more substances that can exist in the gas, liquid, or solid phases. The amount of heat released or absorbed during the dissolving process is referred to as the enthalpy change of solution (at constant pressure). This enthalpy of solution (Solution) can be positive (endothermic) or negative (exothermic) (exothermic). To understand the enthalpy of solution, imagine a hypothetical process where two substances interact to form a third substance. The solute is one substance, which we’ll refer to as A. The other substance is the solvent, which we’ll refer to as B.
Conclusion
Bond enthalpy and reaction enthalpy help us understand how energy is used in a chemical system during reactions. Bond enthalpy describes the amount of energy required to break or form a bond and also serves as a measure of bond strength. It is possible to estimate the total change in potential energy of a system by combining the bond enthalpy values for all of the bonds broken and formed during a reaction. which is ΔHrxn for a reaction at constant pressure. We can determine whether a reaction will be endothermic or exothermic based on whether the enthalpy of the reaction is positive or negative.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





