Introduction
As we know, atoms exist in nature independently, except for noble gasses. A group of atoms has different characteristic properties and is found to exist together. And these groups of atoms form molecules. These molecules are held together by a certain type of force which holds various constituents ions and atoms together in different chemical species called chemical bonds. When these chemical bonds are formed by the combination of atoms of various elements. This situation raises many questions like: How do atoms combine and why? How are these molecules connected to each other? Why do molecules possess definite shapes? Here’s the answer to all the above questions.
Body
In 1916, Kossel and Lewis, gave a satisfactory explanation regarding chemical bonding and metallic bonding. They were the first to give some logical explanation about metallic bonding. According to Lewis postulate, atoms possess a stable octet when linked to one another by chemical bonding.
There are several types of bonds:
1) Electrovalent bond: When positive and negative ions are bonded by electrostatic attraction, then this type of bond is termed as electrovalent bond. And it is also referred to as an ionic bond.
2) Covalent bond: When two atoms share electron pair, the bond created is known as covalent bond
3) Hydrogen bond: A bond formed between hydrogen atoms which is located between a pair of other atoms.
4) Metallic bond: It is formed when atoms of the molecule are held together by a certain type of force in a metallic substance called metallic bonding.
In this article, we are going to discuss in detail about metallic bonding.
What do you understand by metallic bonding?
Before answering this question, we have to learn about metals. We can define metal by its various properties like: Metals are shiny, solid material, ductile, also have good electric conductivity, and have a shining surface called metallic luster. For example: Iron, copper, aluminum, magnesium, lead, zinc etc. Metallic luster occurs between metals when metals are bonded by the electrostatic force between conduction electrons and positively charged metal ions.
Definition – In simple words, metallic bonding is a type of force that holds atoms of various molecules together in a metallic substance.
But, how are they held together? How does the electrostatic attractive force make them stay together? Don’t worry, this article will answer all these questions for you.
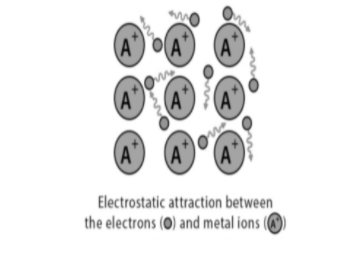
The electrons of the atom leave behind which become a positive ion and the interaction between such positive ions and valence electrons gives the cohesive or binding force which holds the metallic crystal together. Metallic bond is between metal cation and delocalized electron and its nature describes the various physical properties of metal such as conductivity and malleability. Metals can conduct electricity due to delocalized electrons.
As delocalized electrons are able to carry charges, the next question that comes to our mind would be: How do metallic bonds form?
The answer of this question is, charges spread over at a large distance when compared to the size of single atoms in solids. They are so close to each other that valence electrons can be moved away from their atoms. A lattice of positively charged metal ions are surrounded by a “sea” of free delocalized electrons.
Occurrence of Metallic Bonding
It occurs when a metal is in solid state or a liquid state. As we know, the valence electrons of s and p orbitals are loosely packed and this forms a “ sea “ of electrons which surrounds the metal cations. Also these electrons can move throughout the electron sea which means the electron can move freely in these seas.
Here we can say that metallic bonding joins the bulk of atoms and forms various types of molecules. Metals have a high melting and boiling point, forming strong bonds between atoms.
Effects of boiling on Metallic Bond
When we boil a metal, metallic bond is still present but the structure has been broken down because on melting the bond becomes loose not broken. It clearly means that boiling point determines better strength of metallic bonding than the melting point.
The strength of metallic bond depends of these three points:
1) The charge on the cation
2) Size of the metal
3) The number of delocalised electrons which move freely in the electron sea.
NOTE: Stronger the metallic bond, more will be the electrons delocalized. As the nuclear charge on the cation increases, the size of the cation becomes smaller.
Metallic bonds require a great deal of energy because they are strong enough to break. That’s why the metals have high melting and boiling point.
- Metallic bonding has low delta x and low x.
- Where, delta x is the difference in electronegativity.
- Delta x is average electronegativity.
Example of Metallic Bonding
When an aluminum foil comes in contact with the copper wire, you will see a metallic action. As we know, metals have a high melting and boiling point which forms strong bonds between atoms.
- CaCl2 – it also forms metallic compounds by forming metallic bonds.
- Graphene – It is an allotropes of carbon atoms which exhibit two dimensional metallic bonding.
In chemistry,
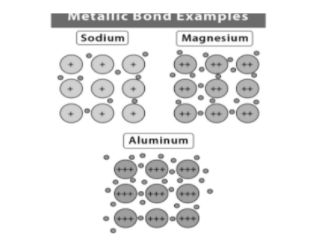
1) Sodium: The outermost electron of sodium metal has lone pair electrons in 3s orbitals. That outermost electron of one atom shares with the corresponding electron on a neighbor atom. This results in the formation of the 3s molecular orbital. Each atom of sodium has eight other atoms in its neighbor. The sharing is between the central atom of sodium and 3s orbital of its neighbor. The outermost electrons are delocalized over the metal structure.
2) Magnesium: As we know, the outermost shell of magnesium loses two electrons which are delocalized. Magnesium has more electron density than sodium because the attraction between nuclei and delocalized bonds is stronger than the sodium. The bond strength is generally higher in magnesium.
3) Aluminum: It possesses three valence electrons in its outermost shell that is 3s and 3p orbital. On the next step, aluminum possesses +3 positive charge when electrons become loose. These ions are held together by negative electrons even though they repel each other. In this way they share the electrons and form metallic bonds by forming crystalline structure.
Properties of Metallic Bonds
1) THERMAL CONDUCTIVITY – Metals conduct electricity because they carry charges on them. When metals are heated the charges become active and start moving in action. Therefore they conduct electricity.
2) MALLEABILITY AND DUCTILITY – Metals are very malleable and ductile, as a thin sheet can form by hammering or rolling of metal whereas ductility is the ability of metal to deform under solid state.
3) HIGH MELTING AND BOILING POINT – Metals possess high melting and boiling point because of formation of metallic bond between sea electrons and the delocalized electron of its neighbor atom.
4) LUSTER AND SHINING – The delocalized electrons absorb and emit visible light. As metals have specific energy levels that’s why some visible radiation is absorbed which gives rise to the shininess of metal. Thus metals impart colors.
METALLIC BONDING STRUCTURE
Conclusion
Hence we conclude that metals form metallic bonds when they are in solid or liquid state. As they can form metallic bonds when they share the sea electrons of the outermost orbital with its neighboring atom. There is a certain type of electrostatic attractive force which makes them stay together. The strength of metals is defined by its metallic bond as this bond is still present when metals boil. We can also say that metals are stronger than non-metals . Metals possess several types of properties which can also be described by metallic bonding. So at last we come to the point that “ a force which makes the atom stay together or held closely in a metallic substance is called metallic bonding.”
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





