The electrochemical sequence is defined as the arrangement of elements or their ions in ascending or descending order of standard electrode potential under standard conditions. The electrode potential is also the reduction potential and is calculated for many factors by comparing it to the standard electrode potential of the hydrogen electrode, also known as the standard hydrogen electrode.
Electrochemical Series Chart Introduction
Suppose the electrodes (metals and non-metals) in contact with those ions are arranged based on their standard reduction potential or standard oxidation potential values. In that case, the resulting sequence is the elemental electricity. It is called a chemical or electrochemical, or active series. A series of standard electrode potentials was established by measuring the potentials of various electrodes compared to the standard hydrogen electrode (SHE).
According to international treaties, the standard potentials of the electrodes to reduce the half-reaction is tabulated, indicating the tendency of the electrodes to behave as the cathode of the Standard Hydrogen Electrode.
An electrode with a positive E ° value for the reduced half-reaction actually acts as the cathode for the Standard Hydrogen Electrode. In contrast, an electrode with a negative E ° value for the reduced half-reaction instead acts as the anode for the Standard Hydrogen Electrode increase.
The electrochemical series is shown in the table below. The standard state potential of the cell is the cell potential under standard state conditions, estimated at a concentration of 1 mol/litre (1M) at 25 ° C and a pressure of 1 atmosphere.
Electrochemical Series Definition
The electrochemical series, sometimes called the activity series, is a list that describes the arrangement of elements in ascending order of electrode potential values. This series was built by measuring the potential of various electrodes and standard hydrogen electrodes.
Electrochemical Series Chart
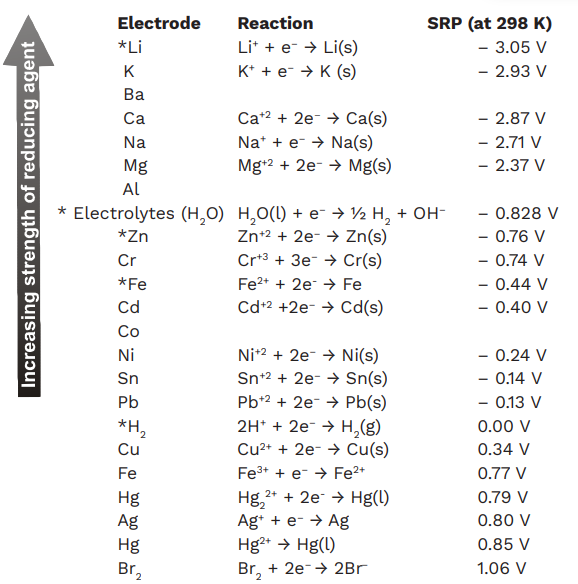
Features of Electrochemical Series
The electrode potential of hydrogen (standard hydrogen potential, SHE) is 0.00 by definition. All other potentials are defined in this context.
Half-cells (element/ion pairs) with a very positive electrode potential are higher in the electrochemical series. They are powerful oxidants.
A negative potential half-cell is a reducing agent—the greater the reducing power, the more negative the value.
Metals are generally electropositive, while non-metals are electronegative. The following metals are the most reactive.
The non-metals at the top of the column are the most active. Therefore, the reactivity is the lowest along the centre. The lower metals of the series can reduce the upper metals of the series. Similarly, non-metals at the top of the series can oxidize metals and non-metals at the bottom of the series.
Important Characteristics of Electrochemical Series
Substances that are stronger reducing agents than hydrogen are ranked above hydrogen and have negative values of standard reduction potentials.
All substances that have positive values of reduction potential and are placed below hydrogen in the series are reducing agents weaker than hydrogen.
Substances that are stronger oxidizing agents than the H+ ion are placed under hydrogen in the series.
Metals at the top (which have high negative values of standard reduction potentials) easily lose electrons. These are active metals.
The non-metals at the bottom (which have high positive values of standard reduction potentials) tend to accept electrons easily. These are active non-metals.
Important of Electrochemical Series
Calculation of Cell EMF
Each electrochemical cell consists of two half-cells on each electrode. Each half-cell undergoes a reaction: oxidation and the other is reduction. Corresponding to every reaction, there are potentials, namely oxidation potentials and reduction potentials.
Cell EMF (Ecell) is the sum of the cell’s oxidation potential and reduction potential. It measures the spontaneity of the entire intracellular reaction. It is also a measure of the work a cell can do. The Electrochemical series helps to measure the EMF of a cell by taking readings of the standard electrode potential of the half-cell and adding them appropriately.
E∘cell = E∘red–E∘oxd
where E∘red is the standard reduction potential of the reducing half-cell, and E∘oxd is the standard reduction potential of the oxidizing half-cell.
Measuring Spontaneity of a Reaction
The feasibility or spontaneity of a redox reaction is directly related to the electromotive force of the corresponding reaction.
If the cell EMF is positive, the response is spontaneous.
If the cell electromotive force is negative, the response is not spontaneous.
Therefore, by examining the reactants and products, it is possible to determine whether the redox reaction occurs spontaneously. Write the equations for both the reduction half-reaction and the oxidation half-reaction. Then, appropriately, add their standard electrode potentials based on the electrochemical sequence.
The resulting cell EMF indicates whether the response is spontaneous.
Estimating Gibbs Free Energy
Gibbs free energy (ΔG∘cell) is another measure of reaction spontaneity. It is related to the cell electromotive force (E∘cell) as follows.
ΔG∘cell = −nFE∘cell
Where n is the number of electrons involved in the reaction and F is the Faraday constant, equal to 96.485 Coulomb-mol-1
Again, based on the EMF code of the cell:
If the cell’s EMF is negative, the Gibbs free energy is positive, and the response is not spontaneous.
If the cell’s EMF is positive, the Gibbs free energy is negative, and the response is spontaneous.
Conclusion
The electrochemical sequence takes the reduction potential of the element with respect to the hydrogen scale, where Eo = zero. The standard definition of the reduction potential of an element is “a measure of the probability that an element will undergo reduction”.
The larger the reduction potential of an element, the easier it is to reduce. On the other hand, elements with a low reduction potential are oxidized much faster and more easily.
Elements with negative or low reduction potentials easily lose electrons.
Elements with positive or higher reduction potentials do not easily lose electrons, but they do acquire electrons easily.
More strong reducing agents with a negative standard reduction potential are usually found in the electrochemical series under hydrogen. However, in this series, a weak
The reducing agent with a positive standard reduction potential is identified above hydrogen.
As you move down the group, the strength of the reducing agent increases, and the strength of the oxidant decreases. Similarly, metal positivity and activity increase or enhance as you move down the series. For non-metals, it decreases.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





