It is possible to determine events that occur on a microscopic scale, such as collisions between individual particles, using kinetics studies of a chemical system, such as the influence of changes in reactant concentrations. The collision model of chemical kinetics, which is a useful tool for understanding the behaviour of reacting chemical species, was developed as a result of these experiments. The collision model explains why chemical reactions occur at higher temperatures more frequently.
For example, a temperature increase of only 10°C doubles the reaction rates of many reactions that occur at ambient temperature. In this section, we’ll look at the relationship between temperature and reaction rates using the collision model.We must first address three microscopic elements that influence the observed macroscopic reaction rates before diving into the link between temperature and reaction rate.
Collision theory of reaction rates
When you heat anything, the particles move quicker. If they move quicker, they will collide more frequently, increasing the chances of a reaction.
The problem with this hypothesis is that it only accounts for a small part of the observed change in reaction rate as temperature rises.
If you raise the temperature by 10°C for a typical response at room temperature, the collision rate barely increases by about 2%. However, the reaction rate will nearly double, representing a 100 percent increase.
Finally, the effect of temperature on reaction rate is determined by its effect on the rate constant k, which is dependent on the reaction’s activation energy Ea. At higher temperatures, a greater proportion of molecules will reach the minimum kinetic energy required to initiate the reaction.
Lets see what Activation Energy is
Study the diagram given below:
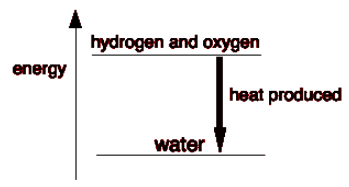
When the gases mix to form water, it is an exothermic reaction that produces a lot of heat. After the hydrogen and oxygen mix, the system becomes more energetically stable.So, if mixing hydrogen and oxygen makes them more stable, why don’t they react right away?Bonds must be broken and new bonds must be established for any response to occur. When bonds are broken, energy is released; when new bonds are formed, energy is released.
To break the bonds between the hydrogen and oxygen molecules in the reaction between hydrogen and oxygen, a lot of energy is required.
Collisions between the molecules in a hydrogen/oxygen combination at typical temperatures do not provide enough energy to do this.
The activation energy is the smallest amount of energy required for a collision to trigger a response.
To demonstrate this, we can change the last diagram. A reaction profile is what this is called. Any exothermic reaction can be represented in the graphic below.

Of course, the activation energy is significantly higher this time.
Temperature and energy
- The average kinetic energy of a substance’s particles is related to its temperature. You’ll notice an increase in temperature if the average kinetic energy rises.
- This is, nevertheless, an average kinetic energy. Individual particles within that may have a very low energy, a moderate energy, or a very high energy and this will change all the time when particles clash.
- At a given temperature, however, the average will remain the same.In terms of reaction rates, what we’re really interested in are particles with high enough energies at the time that when they meet, they achieve activation energy.
- Particles with moderate or low energy will just bump into each other again, causing no effect.
Effect of temperature on reaction rate in terms of activation energy
Collisions must provide energy equal to or higher than activation energy for a reaction to occur. As a result, we’re only interested in particles that have extremely high kinetic energy at the time.
The effect of increasing the temperature on all particles is not proportionate. Instead, it results in a significant rise in the number of active particles.
As a result, the primary effect of temperature on reaction speeds is to increase the number of particles whose collision energies are equal to or greater than the activation energy.
Other factors affecting the reaction rate are
- Reactants concentration
- Physical state of reactants
- Light
- The presence, type and concentration of a catalyst or inhibitor
Conclusion
Chemical reactions can be found all around you and within you. In addition to the biological mechanisms that allow you to convert the molecules you eat and breathe into useful energy, there are industrial laboratories all over the world that produce chemicals and goods that rely on chemicals.
Aside from the product or products generated and having a sufficient supply of reactants, one of the most essential features of a reaction is how quickly it can be expected to proceed. This may have an impact on product quality, safety, and other factors. Temperature is one of the parameters determining reaction speeds that may be easily adjusted in most labs.
Consider how temperature might affect reaction rates, and continue reading to learn more about the numerous factors that might speed up or slow down chemical reactions.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out



