Whenever we consider a liquid, the behaviour of its surface molecules and the rest are entirely different. For example, if you place a small needle on the water’s surface, it will sink to the bottom of the water container. On the other hand, a leaf will float on the surface instead. Such kind of behaviour is explained with the concept of dimensional surface tension. Surface tension explains several behaviours like formation of mercury droplets but splatters of water, the rise of mercury through the thermometer, and such instances. Moreover, this theory forms the base of buoyancy, the Archimedes Principle, and several other facts.
Surface tension – definition and example
There are two definitions for defining surface tension. When liquid is in a container, the top surface molecules contact the air. Due to the interactive forces, the surface molecules behave as an elastic layer. A force higher than the attractive forces between the liquid molecules needs to be applied to break through the surface.
However, this is a simple physical definition that won’t play a significant role in answering dimensional surface tension questions. Therefore, we need to learn the mathematical definition and the formula to understand its dimensional behaviour.
Accordingly, surface tension is the force needed to break the attractive forces between the surface molecules. Mathematically, we can express surface tension as:
γ = Force / length.
Or, γ = f/l.
Therefore, surface tension is the total force applied per unit length of the liquid surface to break the film. Its SI unit is expressed as N/m, while the CGS unit is dyne/cm.
Reasons for surface tension
Surface tension is the result of interactive forces between the liquid molecules. If we consider a liquid kept in a large tub, we have to compare the behaviour of the two molecules. Molecule A is present at the surface, while Molecule B is suspended somewhere between the water layers. In molecule B, all the forces acting on it cancel each other as the components are equal and act in the opposite direction.
However, if we consider the surface molecule, B, an equal amount of cohesive force acts on both sides and the bottom. But the top portion is exposed to air pressure. Therefore, to counteract the atmospheric pressure, the cohesive forces between the surface molecules increase significantly, resulting in surface tension.
The dimensional formula for surface tension
Dimensions are critical in defining the dependency of any dependent physical unit over the independent units, namely length, mass, time, temperature. With this, it becomes more relatable how the parameter will vary per other factors. For example, when we consider velocity, it has nothing to do with mass but only time and distance. Similarly, our body weight is dependent on body mass only and not on the gravitational acceleration, since it is constant.
Therefore, to define the dimensional surface tension, we need to learn about the derivation of its formula. Per the above notes, surface tension is described as:
γ = force / length.
Force is expressed as the product of mass and acceleration. Acceleration is further defined as the rate of velocity per unit time or square rate of displacement per unit time. Combined, the physical expression for force can be stated as:
F = (mass * distance) / time2.
Here, the mass has the dimension of [M], distance is defined as length and has the dimension of [L], and time is defined as [T]. After replacing all these values in the expression of the surface tension, we can write:
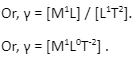
Inference of surface tension
According to the dimensional surface tension questions, the expression of this physical parameter is defined as:
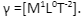
From this, the following facts can be inferred:
- Surface tension is directly proportional to mass, and hence, if mass decreases, the surface tension will also decrease and vice versa.
- Surface tension is directly proportional to length. Therefore, when you want to break a significant film, the tension force needs to be applied in more quantity.
- Surface tension is in direct proportion to time.
Conclusion
According to the dimensional surface tension UPSC notes, this particular phenomenon plays a crucial role in determining the capillary actions of different liquids, the formation of the meniscus, and the concepts of cohesion and adhesion. As this particular physical unit is considered mainly for defining the behaviour of any liquid surface, it is often associated with other phenomena like buoyancy, floatation, and more. Apart from that, from the dimensional formula, you can understand how mass, length, and time vary in surface tension values. Without this inference, it would have been difficult to explain the real-life examples resulting from dimensional surface tension.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





