Diazonium salts are compounds with the general formula R−N2+ X-. R can either be an alkyl or an aryl group. X- holds Cl-, Br-, HSO4-, BF4- etc.
The term combines three words: ‘di’ means ‘two’, ‘azo’ means nitrogen,’ and ‘ium’ means cationic. They are named by suffixing diazonium to the name of the parent alkyl or aryl compound they are formed from and followed by the anion.
Diazonium salts are made up of two nitrogen atoms, one of which is charged. The diazonium salt includes compounds such as benzene diazonium chloride (C6H5N2+Cl-), benzene diazonium hydrogen sulphate (C6H5N2+HSO4-), among others.
Primary aliphatic amines from unstable diazonium salts, while primary aromatic amines form diazonium salts that are stable for a short time in low-temperature solutions ranging from 0 to 4 degrees Celsius.
The most stable diazonium salt are benzene diazonium halides. They are the most stable because of their triple nitrogen bond with the benzene ring.
Importance Of Diazonium Salts In Synthetic Organic Chemistry
Diazonium salts are commonly used chemicals in synthetic organic chemistry.
Initially, diazonium salts were employed to make water fast dyed fabrics, with the materials being immersed in an aqueous solution of the diazonium compound. The coupler, an electron-rich ring that performs electrophilic substitution processes, would then be immersed in a solution.
Today, diazonium salts are widely employed in the pigment and dye industries, primarily in producing coloured fabrics. Diazonium salts are commonly used to create azo dyes. Therefore, they play an essential role in industrial and synthetic organic chemistry.
The majority of azo dyes are made in two-step processes. The aromatic diazonium ion is created in the first step using an aniline derivative. The diazonium salt is then coupled with an aromatic molecule in the next phase. Azo dyes come in various colours, including orange, brown, blue, yellow, and red.
Diazonium compounds, particularly aryl derivatives, are typical reagents in synthesising organic molecules. Because substituted aromatic compounds cannot be generated by direct substitution in benzene, diazo compounds are replaced in the diazonium salts for these compounds.
Diazonium salts are utilised as intermediates to introduce the fluoride, bromide, chloride, iodide, hydroxyl, and -CN groups to the aromatic ring.
General Formula for Preparing Diazonium Salts
Diazonium salts are formed when the primary amine is treated with nitrous acid. As nitrous acid is quite unstable, it is generated by adding sodium nitrite with a strong acid like HCl or H2SO4. This conversion is referred to as diazotisation.
Diazonium salts derived from primary aliphatic amines are unstable even when kept at low temperatures. As a result, they quickly decompose into nitrogen and carbocation. This carbocation undergoes elimination and substitution reactions.
Diazonium Salt Reactions
Diazonium salt reactions are mainly divided into two categories. These categories are based on the use or retention of the diazonium group.
Chemical Reactions of Diazonium Salts
Since the construction blocks for other organic reactions can be synthesised from diazonium salt reactions, so it is necessary to study these reactions. These reactions can be classified as either Sandmeyer reactions or as a result of the use of other mechanisms. Diazonium Salt reactions are as follows:
Type I: Sandmeyer Reaction
An aryl diazonium salt can be converted into an aryl halide by using the Sandmeyer reaction, a radical-nucleophilic aromatic substitution. The Sandmeyer reaction is responsible for many of Benzene’s transformations, including Benzene and halogenation. The nucleophile can be cyanide, thiols, or halide anions, and this method makes it easy to substitute an aromatic amine into copper(I) salts.
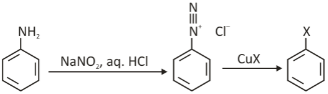
Starting with diazonium salts and copper compounds like copper (I) chloride is a good place to start in these reactions. It is known as the Sandmeyer reaction, when copper (I) chloride reacts with aryl chloride to form copper chloride. There’s a copper (I) chloride reagent discovered in 1884 by Swiss chemist Traugott Sandmeyer that causes a rapid loss of nitrogen from diazonium salts.
The reaction is made up of the following steps:
- To make an arylamine, you must start with its nitro compound, where the benzene ring is deactivated, and electrophilic replacements take place in multiple meta locations.
- Electrophilic substitution occurs at ortho and para locations when the amine’s nitro group is reduced, activating the aromatic ring.
- Aryl amines are converted into diazonium ions, which can then be used in the next-in-line reactions.
Some examples are as follows:
The aryl diazonium salts are converted to aryl chlorides, bromides, and cyanides using CuCl, CuBr, and CuCN.
Type II – Other Reactions
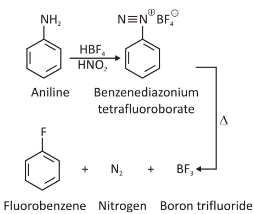
Schiemann Reaction
Aryl fluorides can be formed when the diazonium salt (X;) ion is substituted with the tetrafluoroborate (BF4- ) ion. With heat exposure, stable diazonium tetrafluoroborate salts may become nucleophiles and lose their nitrogen; to yield aryl fluoride and N2 as byproducts, as well as BF3 and BF4 ions. The chemical equation for the Schiemann reaction is shown in the figure below.
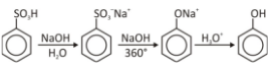
Phenol Synthesis
Aryl diazonium salt (aryl diazonium salt) is heated with water and acid to form hydroxyl groups (OH). In pharmaceuticals and drug development, these phenols are essential building blocks. The underlying chemistry is as follows:
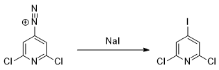
Aryl Iodide
They also react with potassium iodide to produce aryl diazonium salts. The following is the chemical reaction that occurs during the process:
From the Amino Group, Diazonium Salt Is Reduced
When hypophosphorous acid (H3PO2) reacts with aryl diazonium salts, the nitro (amino) group is removed, resulting in C-H. As an example of a chemical reaction, consider the following:
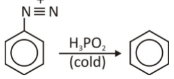
Diazonium Salt Mechanism
Diazonium salt reaction in which diazonium ions are formed through a process called diazotisation, which is similar to the operation of a diazonium salt synthesis. When sodium nitrite (NaNO2) is treated with nitrous acid (HNO2), it becomes sodium nitrate (NaNO2).
When the compound is exposed to HCl, the NaNO2 is converted to HNO2 via the following reaction:
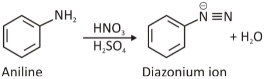
Even more so, the strong acidity of the HCl reduces HNO2 to its electrophile form known as NO+ (nitrosonium), which serves as the building block of the diazonium salt.
When the nitrosonium ion is treated with aromatic amine and a strong acid, the diazonium ion is formed.
Reactions of Benzenediazonium chloride
Benzenediazonium chloride reactions can be divided into two types.
- Reactions resulting from the substitution of another group for the diazonium group
- Diazonium ion coupling reactions
The dye industry relies on benzene diazonium chloride as a raw material.
Diazonium Coupling Reactions
Diazonium salt reaction in which electron-rich nucleophiles can only couple with terminal nitrogen because of the positive charge displacement on nitrogen atoms, resulting in azo dyes. You can visualise this concept by looking at the reaction shown below.
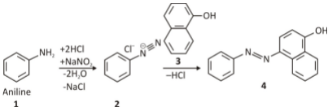
Synthetic dyes such as yellow, red, and orange are made using diazo coupling reactions, which are common in many fields. Because of their ability to affect colour, these compounds are referred to as azo dyes, and they can exist in both cis and transforms.
Physical properties:
Aryl diazonium salts are colourless crystals that turn brown when coming in contact with air. They are unstable compounds that are also highly soluble in water. Furthermore, they are made up of ions.
Conclusion
From the above reactions, it’s clear that diazonium salts act as excellent intermediates for introducing varied groups in aromatic ring structures. This is the reason why they are used within the synthesis of organic compounds. They are primarily used in dye and pigment industries.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





