The depression of the freezing point is a colligative property due to the addition of solute molecules to any solvent. To be very precise, the depression of freezing point is a term that refers to the lowering of the freezing point of solvents due to the addition of solute molecules to it. Due to a decrease in temperature, a substance starts freezing, and its intermolecular forces take over, arranging themselves in a pattern and eventually turning into solid.
Depression in Freezing Point
Let us take an example to have a better understanding. When we keep water to cool, if the temperature is below the freezing point of water, the hydrogen bonding begins to stick more and, thus, result in the formula for the depression of freezing point:
△Tf = i x Kf x m
Here, △Tf stands for depression of freezing point, i stands for Van’t Hoff Factor, Kf stands for cryoscopic constant, and m represents molality.
We can determine the molar mass of a given solute from the above-written formula. Also, we can measure the degree to which a solute dissociates into a solvent.
We can obtain the freezing point of a solution if the chemical potential of a pure liquid solvent reaches that of a pure solid solvent.
The calculation of the molecular mass of a solute using depression in freezing point is as follows:
M = (1000 × Kf × w2) ÷ (∆Tf × w1)
Here, w2 stands for the weight of solute, w1 stands for the weight of solvent, Kf stands for molal depression constant, and ∆Tf stands for depression in the freezing point.
Why Does the Depression of Freezing Point Occur?
There can be many reasons why the depression of the freezing point of a solvent occurs when we add solute molecules to it. Let us take a close look at those reasons:
- There is an equilibrium state between the liquid and solid state of the solvent at its freezing point.
- It means that the vapour phases of liquid and solid are equal.
- When we add non-volatile solute molecules to a solvent, the vapour pressure of the solution goes lower than the vapour pressure of the pure solvent.
Effect on Physical Properties Due to Solutes
To understand the effects of solutes on the physical properties, we need to understand the graph given below:
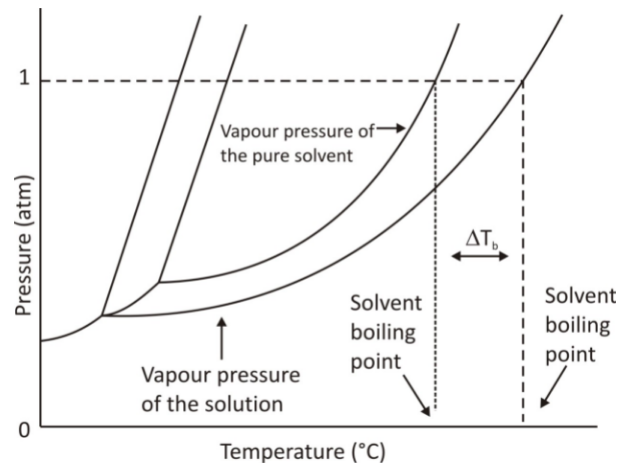
The graph represents the pressure and temperature of the standard boiling point and freezing points of a solvent and the boiling and freezing points of a solution. You can see at 1atm of pressure, the freezing point decreases.
Factors that Affect the Freezing Point
Many factors affect the depression of freezing point, which are as follows:
- Intermolecular forces of attraction: The intermolecular forces of attraction between the molecules of the liquid are directly proportional to its freezing point. Because if the intermolecular forces of attraction between molecules are weaker, the freezing point also gets low.
- Physical and chemical changes: Both physical and chemical changes alter the freezing point of a substance.
- Pressure: Changing the pressure also affects the freezing point of a substance.
Process of Freezing
In the process of freezing, a substance changes its state from liquid to solid. When a substance is in a liquid state, its molecules are loosely bound, and the intermolecular forces are also less than that of solids.
When a substance is in a liquid state, the molecules are in a continuous motion. But when the substance freezes, it loses its thermal energy and comes closer to each other.
The temperature remains the same during the freezing process, and it changes from liquid to crystalline solid state. The energy gets released during this freezing process, as when the molecules are in a liquid state, it is in continuous motion. When it changes to solid, it releases energy.
Examples of Freezing Point Depression
- Freezing point in Vodka: It is a solution of ethanol in water. It has a lower freezing point. Depression is less than water but much higher than pure ethanol.
- Due to the excessive presence of salts in seawater, its freezing point is below 0°C, and therefore, it remains liquid at room temperature below the freezing point of water.
- You can also see many organisms which survive in freezing climates. It is so because they produce compounds like glycerol and sorbitol that help decrease the freezing point of water in their body.
Uses of Depression in Freezing Point
- In cold areas, where the temperature drops below 0°C, sodium chloride (NaCl) is spread over the roads to prevent ice formation. NaCl lowers the freezing point of water. Hence, ice doesn’t accumulate over the road.
- In areas where the atmospheric temperature drops to 18°C, Calcium Chloride (CaCl2) is used instead of sodium chloride. Calcium chloride is associated with three ions which causes more depression in the freezing point of water and helps to melt the ice on roads.
- During cold seasons, there is a chance of the radiator freezing. We use radiator fluids in automobiles. These fluids are generally made of ethylene glycol and water and help prevent the freezing of radiators.
- It is used as a purity analysis device. It is analysed by differential scanning calorimetry. This method is very effective and gives pure results.
- It is used in the dairy industry. This property makes sure that extra water is not there in milk. Milk with a freezing point depression of 0.509°C is considered pure.
- This property is also used in making ice cream. It helps create a freezing mixture by adding NaCl or another salt to lower its melting point.
Conclusion
The depression of the freezing point is a colligative property due to the addition of solute molecules to any solvent. To be very precise, the depression of freezing point is a term that refers to the lowering of the freezing point of solvents due to the addition of solute molecules to it. Due to a decrease in temperature, a substance starts freezing, and its intermolecular forces take over, arranging themselves in a pattern and eventually turning into solid.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





