What is The Critical Temperature of a gas?
The peak temperature at which a substance(gas) remains in a liquid state of matter defines the Critical Temperature of a gas. In other words, matter cannot be liquified at temperatures above its critical temperature, despite applying any amount of pressure on it. It is denoted by the symbol ‘Tc‘.
Only at fixed temperatures do the substances(gasses)liquify. The conversion of gasses into liquids happens by compressing the given gas at a proper temperature. It becomes tricky and challenging to liquefy gasses because they become harder to liquefy as the temperature increases because the kinetic energies of the particles that make up the gas additionally increase. Thus, a substance can only be converted to a liquid state from a gaseous state up to a specific temperature (critical temperature) and not above it.
Heating Above the Critical Temperature
Three results are seen when we start to increase the temperature of a particular substance.
- There is a very high movement of molecules.
- The liquid density drops down
- The vapor pressure rises.
Thus the vapor density goes up. The vapor pressure at some temperatures becomes so high that the vapor density is equal to the density of the liquid. At this point, the vapor and liquid both become identical. This is, of course, the critical temperature. This temperature (critical temperature) is when the density and other properties become the same. The force of attraction acting on the substance becomes insufficient in condensing it to the liquid because of the very high movement of the molecules.
Critical Temperature through Graph
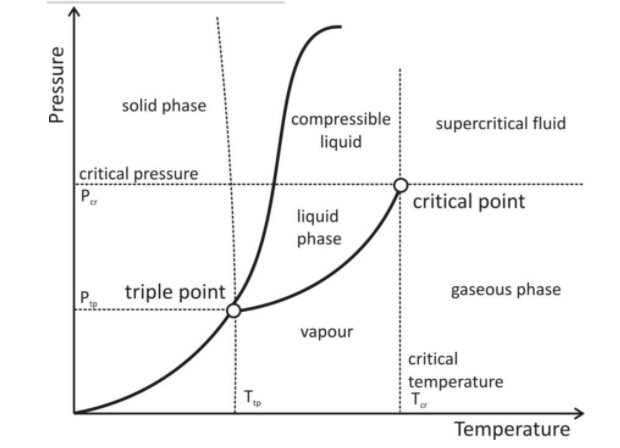
The graph mentioned above shows Temperature and Pressure on the X and Y axes, respectively. In the diagram, the temperature indicates that the critical temperature is calculated and achieved from the value of the X-axis on the given graph and on the other hand, the value of the Y-axis shows the value of pressure needed to achieve the liquid state of the substance at the critical point. When the temperature becomes equal to the critical temperature, this pressure at the critical point is called critical pressure(Pc) of the gas/substance. The pressure acting on the substance/body at the critical point and the temperature being the critical temperature is known as the Critical pressure.
The triple point on the graph is the point when the values of the temperature and pressure of the substance are such that they make the substance exist in all three states of matter.
Critical Pressure and Temperatures Chart
Substance | Critical Temperature (Tc) |
Ammonia (NH3) | 405.5K |
Carbon Dioxide (CO2) | 304.19K |
Nitrogen (N2) | 126.2K |
Water (H2O) | 647.09K |
Helium (He) | 5.19K |
Chlorine (Cl) | 416.9K |
Lithium (Li) | 3220K |
Substance | Critical Pressure (Pc) |
Ammonia (NH3) | 111.3 atm |
Carbon Dioxide (CO2) | 72.8 atm |
Nitrogen (N2) | 33.5 atm |
Water (H2O) | 217.7 atm |
Helium (He) | 2.24 atm |
Chlorine (Cl) | 76 atm |
Lithium (Li) | 652 atm |
Table one depicts a few substances’ critical pressures(Pc), and their critical temperatures are shown in table two. It is visible in the table that Ammonia (NH3) cannot be liquified beyond its critical temperature, which is 405.5K, and this is obtained by applying the critical pressure of 111.3 atm. In general, the metals have very high critical pressure and temperature values. It is also understandable that the noble element Helium is among the substances with the lowest critical pressures and temperatures.
Meaning of critical solution temperature
The temperature at which the mixtures become completely miscible as the temperature is lowered or raised is known as critical solution temperature.
Some Amazing Truths
At the point when water is warmed past its critical temperature that is 647 K, and critical pressure that is 218 atm, then, at that point, it has strange conduct. Over the critical temperature, the difference between the fluid and vaporous conditions of water vanishes, and water turns into a supercritical liquid. The capacity of water to go about as a polar solvent (a dissolving medium) likewise changes when it is exposed to temperature and strain past the critical point. When water is warmed more, the atoms associate with nonpolar particles; supercritical water can be utilized as an ignition mode for annihilating harmful materials to break down nonpolar substances. Oxidation in supercritical water can likewise help obliterate a tremendous assortment of dangerous natural substances, considering the benefit that a supercritical-water reactor is a shut framework. Hence, there are no discharges delivered in the open space.
Conclusion
The critical temperature of a substance/matter is the temperature above which the substance/matter cannot be converted to a liquid state of matter, despite any amount of pressure applied to that substance. In general, the metals have very high critical pressure and temperature values.
Tc is a symbol for the critical temperature. It is provided by- Tc=8a / 27bR
Where R is the gas constant and a and b are Van der Waal’s constants.
For now, have a look at a few Frequently Asked Questions. It will surely help you revise all the topics you read just now. I wish you all the very best.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




