Covalent bonds are a class of synthetic bonds where valence electrons are divided among two molecules, ordinarily two non-metals. The arrangement of a covalent bond permits the non-metals to comply with the octet rule and, along these lines, become more steady. Covalent holding requires a particular direction between molecules to accomplish the cross-over between holding orbitals.
Tetravalency of Carbon
Carbon is a nonmetal in group 14 of the periodic table. The nuclear number of carbon is six, with an electronic arrangement of 2, 4. Along these lines, carbon has four valence electrons in its valence shell. Valence electrons are the electrons in a molecule’s external energy level associated with the synthetic bond arrangement.
Carbon needs four more valence electrons to fill its outermost energy level with eight electrons. A full outer energy level is the most steady course of action of electrons.
Carbon can form four covalent bonds. Covalent bonds are bonds that are formed between nonmetals. In a covalent bond, two molecules share a couple of electrons. By framing four covalent bonds, carbon shares four sets of electrons, filling its outer energy level and accomplishing dependability.
Different Types of Carbon Compounds
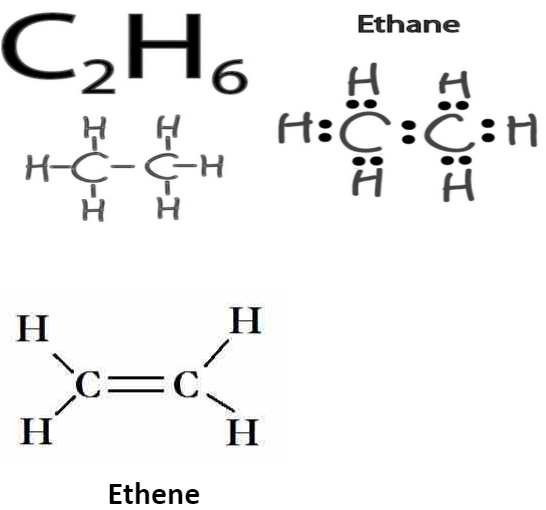
- Saturated carbon compounds: These are compounds in which carbon atoms are linked together with a single bond only to form the aliphatic or ring-like configuration. Alkanes like methane, ethane, etc., are perfect examples.
- Unsaturated carbon compounds: These are compounds in which carbon atoms are linked together with double bonds or triple bonds and single bonds to attain a chain-like or a ring-like configuration. Ethene, benzene, etc., belong to this category of carbon compounds.
Covalent Bonding and Covalent Compounds
A covalent bond is the interatomic bond formed by the sharing of an electron pair between two atoms. The electrostatic attraction of the nuclei of the two atoms for the identical electrons causes the bonding in covalent compounds.
A covalent compound is a molecule produced by covalent bonds in which one or more pairs of valence electrons are shared by the atoms.
Types of Covalent Bonds
All bonds are represented as single lines in the ethane Lewis formula shown.Each bond is made up of two electrons, known as bonding electrons. It is also feasible for two atoms to share four electrons if they are bound together. This bonding structure, known as a double bond, is illustrated by two lines representing two electrons.
An example is the ethene molecule illustrated below.
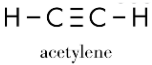
Six electrons can be shared between two atoms. In this situation, the representation is made up of three single lines, a configuration known as a triple bond. The acetylene molecule illustrates a triple bond.
This nomenclature (single, double, or triple bond) is quite flexible and informal. However, calculations do not accurately represent the true nature of the bonds. They are, nonetheless, quite beneficial in a variety of scenarios.
Covalent Polar Bond
Covalent polar bond is formed between the two atoms where the electrons which form the bond are distributed unequally. Electrons will be drawn to more electronegative atoms. As a result, the common electron pair will be closer to that atom. Oxygen-hydrogen, nitrogen-hydrogen, and sulphur-oxygen are molecules that form hydrogen bonds due to an imbalanced electrostatic potential.
Non-polar Covalent Bond
This covalent connection is established when two atoms share an equal number of electrons. Non-polar covalent bonds form when the atoms combining have comparable electron affinities (diatomic elements).
Non-polar covalent bonds, for example, can be present in gas molecules such as hydrogen gas, nitrogen gas, etc.
Covalent Bond Formation
Covalent bonds arise because they provide atoms with a more stable electron configuration. An oxygen atom has six valence electrons on its own. Each oxygen atom contains eight valence electrons since it shares two pairs of valence electrons. This fills its outer energy level, resulting in the most stable electron configuration. The shared electrons are attracted to both oxygen nuclei, and this force of attraction keeps the two atoms in the oxygen molecule together.
Formation of Covalent Bonds in Carbon Compounds
A carbon atom can create covalent connections with other carbon atoms or atoms of other elements. Carbon frequently forms bonds with hydrogen. Hydrocarbons are compounds that include carbon and hydrogen. Methane (CH4) is an example of a hydrocarbon. A single carbon atom forms covalent connections with four hydrogen atoms in methane.
Carbon Compound Bonding
Carbon creates covalent bonds with other atoms in its molecules. Carbon has a valency of four in each compound, indicating that it is tetravalent. Carbon has an atomic number of six, and the first shell has just two electrons, while the outermost shell includes four electrons. The carbon atom can achieve the noble gas configuration by sharing its valence electrons with other carbon atoms or atoms of other elements and forming a covalent bond.
Properties of Covalent Bonds
- They are extremely strong chemical bonds formed between atoms.
- They don’t generate additional electrons. The link just connects them.
- They seldom dissolve spontaneously after formation.
- They are directional, meaning the linked atoms have distinct orientations relative to one another.
- Most covalently bound chemicals have relatively low melting and boiling points.
- Covalently bound compounds often have lower enthalpies of vaporisation and fusion.
- Due to a lack of free electrons, covalent molecules do not conduct electricity.
- Water does not dissolve covalent compounds.
Conclusion
A covalent bond is an attractive force that holds two atoms together that share a pair of valence electrons. Covalent bonding arises between non-metal atoms. A covalent compound is formed when atoms of different elements link together. Since the shared electrons occupy the outer energy level of each atom, covalent connections develop.
This is the most stable electron configuration. Carbon has a high degree of catenation, forming covalent connections with carbon atoms or other elements. Since carbon has four valence electrons, it may make four covalent bonds to obtain a complete outer energy level. It forms compounds known as hydrocarbons when it solely binds with hydrogen. Carbon may create covalent bonds with other carbons in single, double, or triple forms.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





