What is hybridisation?
Pauling introduced hybridisation to explain the equal nature of covalent bonds in a molecule.
- Mixing of different shapes and approximate equal energy atomic orbitals, and redistribution of energy to form new orbitals of the same shape and same energy. These new orbitals are called hybrid orbitals, and the phenomenon is called hybridisation.
Characteristics of hybridisation
- Hybridisation is a mixing/blending of orbitals and not electrons. Therefore in hybridisation, full-filled, half-filled and empty orbitals may take part.
- The number of the hybrid orbitals formed is always equivalent to the number of atomic orbitals which have taken part in the process of hybridisation.
- Each hybrid orbital has two lobes. One is larger and the other is smaller. Bonds will be formed from a large lobe.
i) The 1st bond between the two atoms will be 𝜎.
ii) The other bond between the same two atoms will be a π bond.
iii) The electron pair of an atom which does not take part in bond formation is called a lone pair of electrons.
4. One element can represent many hybridisation states depending on experimental conditions. For example; C showing sp, sp2 and sp3 hybridisation in its compounds.
5. Hybrid orbitals are differentiated as sp, sp2, sp3 etc.
6. The repulsion between lp-lp > lp-bp > bp-bp.
7. The directional properties in hybrid orbitals are more than the atomic orbitals. Therefore hybrid orbitals form stronger sigma bonds. The directional property of different hybrid orbitals will be in the following order.
sp < sp2 < sp3 < sp3d < sp3d2 < sp3d3
Determination of hybridisation state:
METHOD (I): Number of hybrid orbital = number of ‘𝜎’ bond + number of lone pair [surrounding the central atom].
METHOD (II): To predict following formulae may be used:
No. of hybrid orbital= 12[ Ve+SA+C]
[ Ve= total no. of valence electrons in the central atom, SA = total no.of monovalent atom; C = charge]
Eg.
NH4+ 125+4-1=4 sp3 hybridisation
SF4 12 6+4=5 sp3d hybridisation
NO3- 12 5+1=3 sp2 hybridisation
If such type of electron pairs are-
Two – sp hybridisation
Three – sp2 hybridisation
Four – sp3 hybridisation
Five – sp3d hybridisation
Six – sp3d2 hybridisation
Seven – sp3d3 hybridisation
Position of lone pair and multiple bonds.
- sp/sp2/sp3 = anywhere
- sp3d = equatorial
- sp3d2 = axial ( defined first )
- sp3d3 = if lone pair is equal to 1 then; equatorial
if lone pair is equal to 2, then; axial
- In sp3d hybridisation Axial bond > equatorial bond length
In sp3d3 hybridisation Axial bond < equatorial bond length
[NOTE: this applies when terminal atom is same]
Types of hybridisation
A) sp hybridisation :
a) In this hybridisation one s-and one p- orbital of an atom are combined to give two new hybrid orbitals which are equivalent in shape and energy known as sp hybrid orbitals.
b) These two sp hybrid orbitals are arranged in a straight line and at bond angle 180⁰.
c) s-character 50%
for example;
CO2 Molecule (O=C=O):
In the CO2 molecule, C has two sp hybrid orbitals and two unhybridized p orbitals.
Thus, CO2 molecule is linear in shape and has a 180⁰ bond angle.
The bond length between C-O bond is reduced due to the presence of π bond.
In CHCH molecules, each C atom contains two sp hybrid orbitals and two unhybridized p orbitals.
- sp hybrid orbital of each C overlaps to give sigma bond between C-C.
- The remaining one sp hybrid orbital of each C atom overlaps with s orbital of H, forming a sigma bond between C-H.
- The two unhybridized p orbitals of each C atom (py and px) overlap laterally to form two π bonds.
B) Sp2 hybridisation:
a) In this hybridisation one s and two p orbitals are combined to give three new sp2 hybrid orbitals which are in the same shape and equivalent energies.
b) These three sp2 hybrid orbitals are at an angle of 120⁰ and give trigonal planar shape.
c) s – character 33.3%
- SnX2 having two 𝜎 bonds and one l.p. electron therefore hybridisation is sp2.
- The bond angle in SnX2 will be less than 120⁰ (due to presence of one lone pair of electrons).
- The shape of SnX2 molecule is bent.
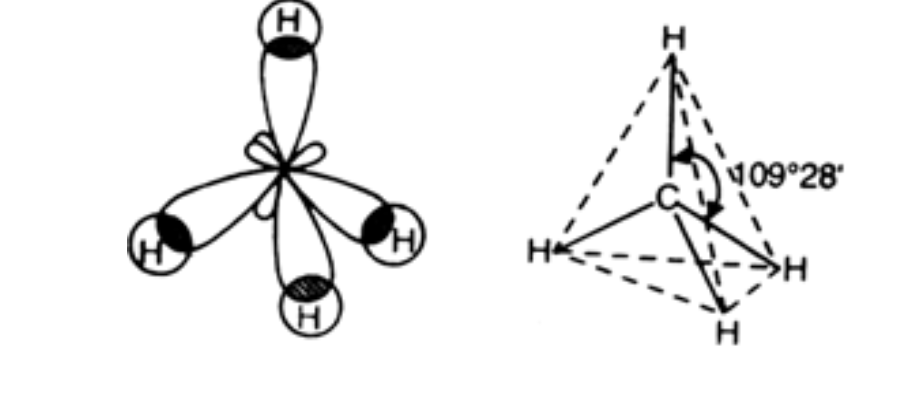
C) Sp3 hybridisation:
In this hybridisation one s orbital and three p orbitals of an atom of a molecule or ion, are combined to give four new hybrid orbitals called sp3 hybrid orbitals.
I) The angle between hybrid orbitals 109⁰28’
The C atom shares four electrons with four hydrogen atoms.
II) The shape obtained from these hybrid orbitals would be tetrahedron.
D) Sp3d hybridisation:
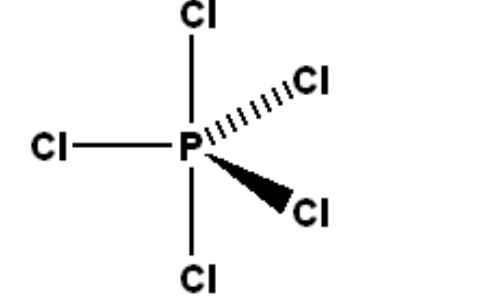
I) In this hybridisation, one s orbital, three p orbitals and one d orbital are combined to give five new hybrid orbitals that are equivalent in shape and energy called sp3d hybrid orbitals.
II) Out of these five hybrid orbitals, three hybrid orbitals are at 120⁰ angle and two molecules becomes trigonal hybrid orbitals are perpendicular to the plane of three hybrid orbitals that is trigonal planar, bipyramidal.
For example, PCl5 showing sp3d hybridisation

III) In this hybridisation dx2 orbital is hybridized with s and p orbitals.
IV) In this way five sp3d orbitals form five sigma bond with five Cl atoms and give a molecule of PCl5, shape of this molecule is trigonal bipyramidal
V) Two axial P-Cl bonds are longer than three equatorial P-Cl bonds due to repulsion between 3 equitorial bp of electrons and 2 axial b.p. of electrons.
E) sp3d2hybridisation:
a) In this hybridisation, one s-orbital, three p-orbital and two d-orbitals (dz2, dx2-y2) are mixed to give six new hybrid orbitals known as sp3d2 hybrid orbitals.
b) The geometry of molecules obtained from above six hybrid orbitals will be symmetrical octahedral or square bipyramidal.
c) The angle between all hybridorbitals will be 90⁰.
Example: SF6, AlF6-3, PF6-, ICl5, XeF4 .
d) Two ‘d’ orbitals participatein the hybridisation are dx2y2 and dx2.
For e.g. SF6
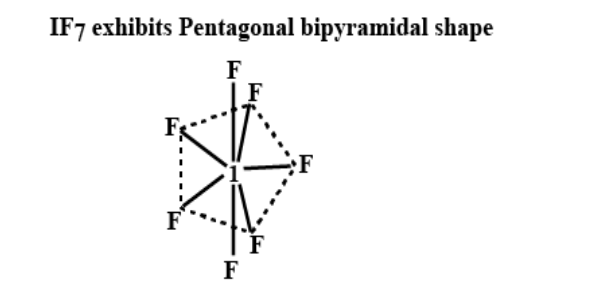
F. sp3d3 hybridisation:
a) In this hybridisation one s- orbital, three p-orbitals and three d-orbitals are mixed to give seven new hybrid orbitals known as sp3d3 hybrid orbitals.
b) These seven sp3d3 orbitals are configured in pentagonal bipyramidal shape.
c) Five bonds are of 72⁰ and 10 bond angles of 90⁰.
d) IF7 and XeF6 are some of the examples showing sp3d3 hybridisation.
Conclusion
In this text, we discovered the concept of hybridisation, like hybridisation, its important features, and the types of hybridisation. We have seen different types of hybridisation like sp3, sp2, sp3d, sp3d2 and sp3d3, along with some of their examples. After studying this article, you will determine the shape hybridisation of any given molecule. To better understand this chapter, other topics such as crystal field splitting theory and crystal field splitting energy are suggested to the user.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




