They can be defined as any type of chemical compound in which one or more carbon atoms, which can be either one or more than one, are covalently linked to the atoms of the other elements. To put it simply, organic compounds are any chemical compounds that contain carbon. However, this does not imply that every carbon-containing compound can be classified as organic, for example, cyanides, carbonates, and carbides are not classified as organic.
Methane is the most basic organic compound and serves as a good example of how simple organic compounds can be. Organic compounds include cyclohexane, ethyne, ethane, and ethane ethers, to name a few examples.
Classification of Organic Compounds
Organic compounds can be divided into two groups based on their structure. As a starting point, we will discuss organic compounds that are based on “Structure,” and then we will move on to discuss organic compounds that are based on “Function.” We will now go over each of these organic compound classifications in greater detail.
- Classification based on the structure
Let us look at the classification of organic compounds based on structure.
- Acyclic or open chain compounds
- Cyclic or closed chain compounds
Acyclic or Open-chain Compounds
Acyclic compounds are diametrically opposed to cyclic compounds in that their molecules do not form any kind of ring structure. Because they have a linear structure, they are referred to as open-chain compounds. Acyclic aliphatic compounds and alkanes are two of the most prominent examples of these compounds. Straight-chain compounds and branched-chain compounds are two types of open-chain compounds that can be understood. Straight-chain compounds do not contain any side chains, whereas the atoms of branched-chain compounds contain the straight chain as well as one or more side chains that are attached to the straight chain.
Cyclic or Closed-chain Compounds
When compared to cyclic compounds, acyclic compounds are diametrically opposed in that their molecules do not form any kind of ring structure. In recognition of their linear structure, open-chain compounds are often used to describe these substances. Two of the most notable examples of these compounds are acyclic aliphatic compounds and alkanes, which are both found in high concentrations in nature. It is possible to understand two types of open-chain compounds: straight-chain compounds and branched-chain compounds. Straight-chain compounds and branched-chain compounds are both types of open-chain compounds. Compounds with straight chains do not contain any side chains; however, the atoms of compounds with branched chains do contain a straight chain as well as one or more side chains that are linked to the straight chain.
Heterocyclic compounds
Histidine-containing heterocyclic compounds are cyclic compounds that contain a structure with a ring at the centre. We can understand these compounds with a very simple definition: they are similar to any other dominant branch of organic compounds in which two or more atoms join in a ring shape in their molecules, as is the case with any other dominant branch of organic compounds. Despite the fact that they contain carbon atoms, it should be noted that they also contain at least one atom of another element. Synthetic dyes, nucleic acids, and the majority of pharmaceuticals are all examples of these compounds that are widely used.
Homocyclic compounds
When it comes to organic chemistry, homocyclic compounds are the type of cyclic compounds that are formed by the atoms, as opposed to heterocyclic compounds, which are formed by the ring structure of the compound. This ring structure is composed of the same elements’ atoms as the previous one, and the element in question is carbon. Carbocyclic compounds are what these are referred to as. There can be no other element present in this compound besides carbon. Although, in inorganic chemistry, homocyclic compounds have ring structures that have been formed by atoms of different elements such as boron, sulphur, phosphorus, and so on, in organic chemistry, homocyclic compounds do not have ring structures. Among the best examples of this compound are naphthalene, tetracene, benzene, and other related compounds.
Heterocyclic Compounds
Heterocyclic compounds are divided into two broad categories, which are named Alicyclic heterocyclic compounds and Aromatic heterocyclic compounds. Now, we will discuss these categories in brief.
Classification of Heterocyclic Compounds
Alicyclic Heterocyclic Compounds
These compounds’ ring structures contain one or more heteroatoms, depending on the compound. Tetrahydrothiophene, tetrahydrofuran, and other similar compounds serve as good examples of how we can understand these compounds.

Aromatic Heterocyclic Compounds
These compounds are easily defined by a simple understanding of their chemistry. In contrast to alicyclic heterocyclic compounds, the molecules of these compounds contain one or more heteroatoms, depending on the type of compound. Thiophene, furan, and other similar compounds are examples of these compounds.
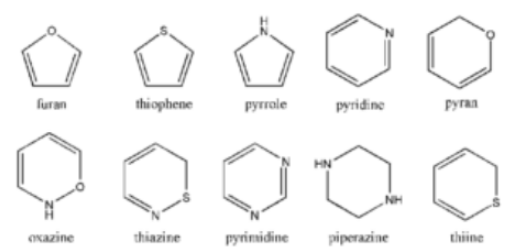
Homocyclic or Carbocyclic Compounds
Compounds that are homocyclic or carbocyclic in nature can be divided into two categories. The first of these compounds is referred to as an Alicyclic compound, and the second is referred to as an Aromatic compound. We will now go into greater detail about these compounds.
Classification of Homocyclic compounds
Alicyclic Compounds
It is an alicyclic compound when it is both aliphatic and cyclic at the same time. This compound contains one or more all-carbon rings, which can be saturated or unsaturated, depending on the amount of carbon present. The bonds that exist between atoms in pairs can be any type of bond, including single, double, and triple bonds.
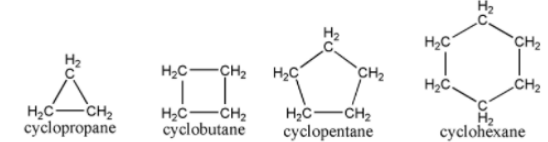
Aromatic Compounds
Aromatic compounds are cyclic compounds that are unsaturated, as opposed to alicyclic compounds, which can be either saturated or unsaturated. Alicyclic compounds can be classified as either saturated or unsaturated. Aromatic compounds, also known as arenas, are a type of organic compound. As their Greek name “aroma” suggests, they have a pleasant odour, which is why they are so popular. Each of these compounds is distinguished by the presence of one or more than one planar ring of atoms that are linked together by covalent bonds of two distinct types. Benzene and toluene are just a couple of examples of these compounds.
Classification of Aromatic Compounds
Due to their exceptional stability, aromatic compounds are referred to as aromatic compounds, which is primarily concerned with odour. We will now discuss the classification of these compounds, which are divided into two groups: Benzenoid Aromatic Compounds and Non-Benzenoid Aromatic Compounds. We will go over them in greater detail later on.
Benzenoid Aromatic Compounds
These aromatic compounds are primarily derived from the chemical compound benzene. The presence of one or more than one isolated or fused benzene rings and their derivatives in the structure of these compounds distinguishes them from other compounds of the same type. According to the number of benzene rings that are fused together in the structure, these compounds can be divided into three categories: monocyclic, bicyclic, and tricyclic benzene rings. When two or more rings are present in the structure of a bicyclic or tricyclic compound, it is said to be tricyclic or bicyclic. Phenanthrene, Naphthalene, and Anthracene are just a few of the compounds that fall into this category. Additionally, a few of these compounds are depicted in the following section.
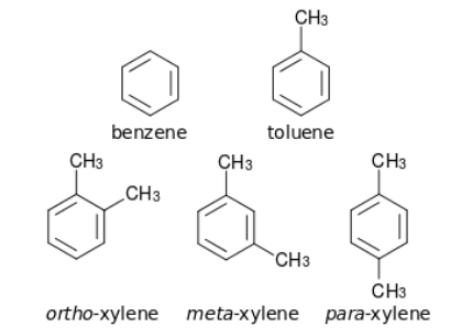
Non-benzenoid Aromatic Compounds
Instead of benzene rings, these aromatic compounds are made up of other unsaturated ring structures. These aromatic compounds have a high degree of chemical stability. Tropolone and Azulene are just a couple of examples of these compounds.
Classification based on the functional groups
According to one definition, “functional grouping” can be defined as the process by which a molecule obtains its characteristic chemical properties from one or a group of atoms that are present in the molecule. To understand why we must classify organic compounds on the basis of functional groups, we must first understand what they are. The answer is very simple because it categorises the chemical behaviour of an organic compound into different groups. We must recognise that the nature of functional groups has an impact on the reactions of compounds, as well as, to a lesser extent, on the physical properties of those compounds.
There are numerous organic reactions that involve the transformation of functional groups and have no effect on the rest of the molecules in the reaction system. It is impossible to list all of the examples of functional groups, but a few examples include the carboxylic acid group (-COOH), the hydroxyl group (-OH), and the aldehyde group (-CHO).
Conclusion:
Alcohols are chemicals that include substances such as ethanol and isopropanol. Their antiseptic properties make them useful in the medical field, and ethanol is a key ingredient in the beverage industry. Carboxylic acids are used in a variety of chemicals, including pharmaceuticals. Carboxylic acid is found in aspirin, which is one of the world’s oldest commercial medicines.
However, despite the fact that there are millions of different organic compounds, there is a fairly straightforward classification scheme for these compounds, as well as an established method for naming even the most complex organic compounds. As a result, you will be able to distinguish between organic compounds and their classifications, as well as name a few of the most common of these compounds, after completing this unit.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out



