Salt analysis is the separation of distinct anions and cations in inorganic salts and the identification of the same. Qualitative analysis of dissolved salts or systematic qualitative analysis is used to describe this method. Inorganic salts are separated into different ions using various laboratory procedures and putting the compounds through various tests to establish if particular ions are present or not in the solution.
Salt Analysis is accomplished by relying on the results to validate the presence or absence of certain cations and anions. To determine the anion of the salt, preliminary testing for anions is carried out group-by-group. After a positive preliminary test for an anion, a confirmatory test is required to ensure that the anion is present in the salt. You must perform a preliminary cation test in salt analysis to check for the presence of various cations in the inorganic salt. This is done in the same way as early testing for anions is done.
How to Analyze Salt
- Determine how much salt you want to put on the exam.
- Determine which anion group is present in NaCl, which stands for salt. Finding a large ion group for most of these studies is simple since there is a typical repetition in groups that results in a positive test result.
- Once you’ve identified a group, test the strength of each anion inside it.
- Carry out the same group-by-group test for cations that you performed for anions.
- Once you’ve identified a group, test the strength of each cation in that group.
- When cation and anion are discovered and synthesized, a chemical formula is created that records the valence of each ion.
- For example, if the cation is Fe3 + and the anion is Cl-, the inanimate salt’s final product will be FeCl3.
- It’s not unusual for anions to be discovered initially. You may also alter the sequence in which each ion is received. Each ion has a distinct reaction to an additional chemical in a confirmatory test, such as the development of a precipitate.
Colors associated with some of the most frequent cations
While analyzing the salt, here are the salt analysis chart of different colors associated with the cations:
Colour of salt | Cation present |
Dark green or purple | Cr3+ |
Deep blue | Co2+ |
Yellow or yellowish-brown | Fe3+ |
Pale pink | Mn2+ |
blue | Cu2+ |
If the salt is colorless, the first step is to conduct a flame test (since the presence of 3 different cations can be confirmed by it). To make the flame test more convenient, you may use test tube holders to hold a lump of salt, which can then be exposed to the flame of a Bunsen burner.
After doing a flame test (or visual examination) and failing to acquire any insight into the cation, you should conduct preliminary testing for cations on each group separately. As shown below, it is important to note that certain cations do not form salts with certain anions.
Ba2+, Sr2+, Pb2+, and Ca2+ do not combine with the sulfate anion to create salts (SO42-).
When combined with the phosphate anion, only the cations from groups 0, 1, and 2 produce salts (PO43-).
If you find one of these cations in the salt analysis, you will not need to run further tests to determine whether or not the associated anions are present.
Finally, some salts are particularly prevalent in salt analysis studies, and they include the following: CaCl2 is the most frequent sodium chloride salt having the bromide ion (Br–), while NH4Br is the most common chloride salt holding the calcium cation (Ca2+), to name a few of examples. Additionally, certain salts may be distinguished by the texture and appearance of their crystals (for example, calcium carbonate has the texture of powdered chalk). For this reason, visiting your chemistry laboratory and visually seeing the salts may assist you in determining the salt’s identity fast during the practical assessment.
Product with a common ion action and solubility
An effect that occurs when two compounds that both ionize to produce the same (common) ion are engaged in a chemical equilibrium is referred to as the common ion effect.
It is less soluble in water when dissolved in a solution that includes an ion that is similar to that of the salt being dissolved in. For example, if a solution of sodium chloride is added to a suspension of silver chloride in water, the solubility of silver chloride in water is decreased.
Product relating to solubility
An expression for the solubility equilibrium constant of salt is composed of the product of the concentrations of the ions, with each concentration increased to a power equal to the coefficient of that ion in the balanced equation for solubility equilibrium.
- Precipitation happens when the ionic product exceeds the solubility product.
- If the ionic product is greater than the solubility product, Precipitation does not occur.
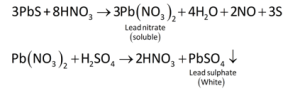
Lead nitrate salt analysis
Due to the generation of soluble lead nitrate, the black PbS precipitate generated in the group analysis dissolves in 50 percent nitric acid. Lead sulfate precipitates when sulphuric acid is added to soluble lead nitrate.
Conclusion:
Analytical chemistry’s qualitative analysis approach is used to determine the elemental composition of inorganic salts. It is primarily focused on detecting ions in a salt aqueous solution. The quantitative and qualitative measurement of cations and anions contained in an inorganic salt is known as salt analysis. Salt analysis entails several procedures that aid in the identification of salt and its component ions. Under laboratory circumstances, the provided salt sample is put through several preliminary and confirmatory tests to assist in differentiating and separating the ions.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





